here - TeacherWeb
advertisement

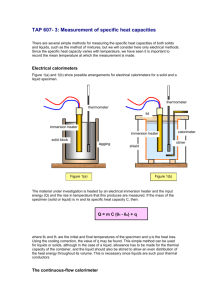
Calorimeter Experiment: by Elizabeth Potoe and Andrew Van Alstyne Prelab: In this lab, you will be measuring the temperature of a reaction in water, and then using that to get the Heat of reaction (qrxn). The reaction is a portion of a flameless heater from an military issue MRE being put into water, and then using the temperature increase to calculate the heat of reaction. The MRE heater is a powder made up of iron, magnesium metal, and table salt. When water is added to this mixture, the result is an oxidation-reduction reaction between the magnesium metal and the water. The reaction for this is unbalanced, you will need to balance the equation. Mg + H2O -> Mg(OH)2 + H2 + heat This reaction on its own is to slow to generate any significant amount of heat, so iron particles and magnesium particles are added to increase the rate of reaction. When water is added to this mixture, it dissolves the salt to create a salt-water electrolyte, which turns each particle of metal into a tiny battery. Because the particles are in contact with each other, these batteries will quickly short circuit and burn out, creating a heat through a process called “Supercorroding Galvanic Cells”. To get the heat of reaction, you first need to solve for the heat of reservoir (qres). The equation you will use is qres = C x mass x ∆T. The value you will use for heat capacity is 4.18 J/g x K, which is the heat capacity of water. ∆T is the difference of the temperature of the water before and after the reaction. The equation for ∆T is ∆T = Tfinal – Tinitial. You will need to convert your temperature readings to Kelvin. After that, you need to find the mass. Your mass measurement will be the weight of the water plus the weight of the reacting powder. Once you have this measurement and the other two, multiply them to get qres. Total mass = _______(grams of water) + _______(grams of powder) C = 4.18 J/g x K ∆T = ______(Tfinal) – ______(Tinitial) qres = ______(C) x _______(mass) x _______(∆T) Once you have qres, you will need to find qrxn, the heat of reaction. To get this, take the negative of the heat of reservoir. qrxn = -qrsn Sample Problem: 1.5 g of powder was added to 100 ml of distilled water. The initial temperature was 19 ⁰C, and the final temperature was 30 ⁰C. What is the heat of reaction? (Hint: 1 g = 1 ml) Procedure: Step 1: Gather materials Two Styrofoam cups, temperature probe, calculator, MRE heater strip, beaker for water, plastic weigh boat, distilled water. Step 2: Measure beaker on a scale, then measure out approximately 100 ml of distilled water into the beaker. Record precise weight. Step 3: Cut MRE heater strip in half, keep one half while giving the other to the next group. Step 4: Measure plastic weigh boat, then pour the MRE heater powder in weigh boat. Measure weight and record. Be careful not to inhale product, or touch face after this point. Step 5: Construct calorimeter. Cut approximately 3/4 of a Styrofoam cup of, use the remaining 1/4 as lid for container. Poke a hole in the lid to allow temperature probe to record. Step 6: Put the measured amount of water in the calorimeter. Record temperature of the water. Step 7: Add the MRE heater strip to the water and cover with the lid. Insert temperature probe to record temperature. Mix contents manually by hand by softly shaking the cup. Step 8: Wait for the temperature to peak, then record temperature and remove temperature probe. Total mass = _______(weight of water) + _______(weight of powder) C = 4.18 J/g x K ∆T = ______(Tfinal) – ______(Tinitial) qres = ______(C) x _______(mass) x _______(∆T) qrxn = -qrsn = ________ Post-Lab: a standard MRE heater has eight half-strips. How much heat would this heater generate? Clean-up Procedure: Step 1: Wash temperature probe under faucet, dry with paper towel. Step 2: Without removing lid, take the calorimeter to pre-arraigned area for disposal. Do not dump contents into a sink. Also take beakers and plastic weigh boats to same location. Safety Precautions: Keep powder away from face, avoid facial contact. Do not handle the powder any more than necessary. Wash hands thoroughly afterwards. If powder comes in contact with eyes, wash immediately. Grading Rubric Pre-Lab Questions 3 Correctly answers all of the questions and shows their work. 2 Sets up the questions correctly and gets most of them correct. 1 Answers some of the questions correctly, but doesn’t show set up. Lab Set Up Correctly sets up the lab, demonstrates understanding of lab set up. Sets up Calorimeter properly, but doesn’t prepare as thoroughly for measurements. Lab Procedure Gathers all data. Lab Data Calculations Can accurately calculate qrxn from lab data. Gathers most of the data Can approximately calculate qrxn from lab data. Sup-par job at setting up Calorimeter, and isn’t prepared to measure materials properly. Gathers some of the data Has to guess qrxn from lab data. 0 Gets most if not all of the questions wrong, and doesn’t set up questions properly. Calorimeter isn’t usable, group doesn’t set up lab. Gathers little or no data Doesn’t calculate qrxn.