PowerPoint - 埼玉医科大学総合医療センター 内分泌・糖尿病内科
advertisement

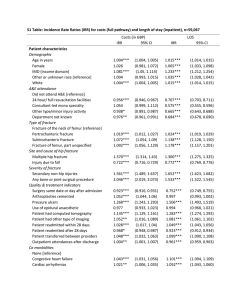
Journal Club Blonde L, Jendle J, Gross J, Woo V, Jiang H, Fahrbach JL, Milicevic Z. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015 May 23;385(9982):2057-66. doi: 10.1016/S0140-6736(15)60936-9. Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR, Wirth CD, Peeters RP, Åsvold BO, den Elzen WP, Luben RN, Imaizumi M, Bremner AP, Gogakos A, Eastell R, Kearney PM, Strotmeyer ES, Wallace ER, Hoff M, Ceresini G, Rivadeneira F, Uitterlinden AG, Stott DJ, Westendorp RG, Khaw KT, Langhammer A, Ferrucci L, Gussekloo J, Williams GR, Walsh JP, Jüni P, Aujesky D, Rodondi N; Thyroid Studies Collaboration. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA. 2015 May 26;313(20):2055-65. doi: 10.1001/jama.2015.5161. 2015年6月18日 8:30-8:55 8階 医局 埼玉医科大学 総合医療センター 内分泌・糖尿病内科 Department of Endocrinology and Diabetes, Saitama Medical Center, Saitama Medical University 松田 昌文 Matsuda, Masafumi The N-terminal amino acid sequence of native human GLP-1 is shown in light green (centre) and the site of cleavage by DPP4 is indicated. The panels show strategies employed to develop a | exenatide and lixisenatide; b | taspoglutide; c | albiglutide and dulaglutide; d | liraglutide and e | exenatide-LAR. Note that development of taspoglutide was halted in 2010, owing to an increased incidence of adverse gastrointestinal effects and hypersensitivity reactions. Abbreviations: DPP4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; LAR, longacting release. Image from the RCSB PDB (www.pdb.org) of PDB ID 1AO6 ( Sugio, S. et al. Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. 12, 439–446 [1999]). Nature Reviews Endocrinology 8, 728-742 a | Native GLP-1 is secreted from intestinal L cells and acts directly on the pancreas to stimulate insulin release and suppress glucagon secretion. GLP-1 inhibits gastric motility, retards gastric emptying and, through actions on the CNS, reduces appetite but can induce nausea. b | Short-acting GLP-1 receptor agonists inhibit gastric motility, reducing transpyloric flow (solid lines). These effects lead to delayed intestinal glucose absorption and, indirectly, to a reduction in postprandial insulin secretion, as well as appetite suppression and induction of nausea (dashed lines). Short-acting GLP-1 receptor agonists also seem to have direct effects on the CNS and on glucagon secretion. c | Long-acting GLP-1 receptor agonists act directly on the pancreas to stimulate insulin secretion, and they suppress glucagon secretion via paracrine release of somatostatin. Through actions on the CNS, these agents also reduce appetite and might induce nausea. Nature Reviews Endocrinology 8, 728-742 Nature Reviews Endocrinology 8, 728-742 The two previous studies of GLP-1 receptor agonists comparing once-weekly with once-daily dosing, DURATION-6 and HARMONY-7 (comparing liraglutide with exenatide once-weekly and with albiglutide, respectively) had non-inferiority margins of 0·25% and 0·30%, respectively, with sample sizes of 911 and 812 patients, compared with 599 patients in AWARD-6. Findings from these studies showed between-treatment group differences of 0·21% (95% CI 0·08–0·33) for DURATION-6 and 0·21% (0·08–0·34) for HARMONY-7, which did not show noninferiority to liraglutide. JB Buse, M Nauck, T Forst, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study Lancet, 381 (2013), pp. 117–124 RE Pratley, MA Nauck, AH Barnett, for the HARMONY 7 study group, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study Lancet Diabetes Endocrinol, 2 (2014), pp. 289–297 AWARD-6 for movie http://www.medscape.com/viewarticle/826899#2 Dulaglutide Weekly Injection (LY2189265) a The Ohio State University, Columbus, OH, USA b Clínica Juaneda, Endocrinología, Palma de Mallorca, Spain c Profil Mainz GmbH & Co KG, Mainz, Germany d Universidad Autónoma de Nuevo León, Monterrey, NL, Mexico e Lilly Diabetes, Eli Lilly and Company, Indianapolis, IN, USA Lancet. 2014 Jul 10. pii: S0140-6736(14)60976-4. doi: 10.1016/S0140-6736(14)609764. [Epub ahead of print] Figure 2: Trial outcome measures (A) Change in HbA1c from baseline to week 26 (MMRM). (B) HbA1c values from baseline to week 26 (MMRM). (C) Percentage of patients achieving HbA1c targets. (D) Change in fasting plasma glucose concentrations from baseline to week 26, as measured by a central laboratory. (E) Seven-point self-measured plasma glucose by time of day. (F) Bodyweight from baseline to 26 weeks (MMRM). HbA1c=glycated haemoglobin. MMRM=mixed model for repeated measures. LSM=least-squares mean. FSG=fasting serum glucose. SMPG=seven-point selfmeasured glucose. *p<0·05. •a Department of Endocrinology, Ochsner Medical Center, New Orleans, LA, USA •b Endocrine and Diabetes Center, Karlstad Hospital, Örebro University, Örebro, Sweden •c Federal University of Rio Grande do Sul, Porto Alegre, Brazil •d Section of Endocrinology and Metabolism, University of Manitoba, Winnipeg, MB, Canada •e Lilly Diabetes, Eli Lilly and Company, Indianapolis, IN, USA •f Lilly Research Laboratories, Vienna, Austria Lancet. 2015 May 23;385(9982):2057-66. doi: 10.1016/S0140-6736(15)60936-9. Background For patients with type 2 diabetes who do not achieve target glycaemic control with conventional insulin treatment, advancing to a basal–bolus insulin regimen is often recommended. We aimed to compare the efficacy and safety of long-acting glucagon-like peptide-1 receptor agonist dulaglutide with that of insulin glargine, both combined with prandial insulin lispro, in patients with type 2 diabetes. Methods We did this 52 week, randomised, open-label, phase 3, noninferiority trial at 105 study sites in 15 countries. Patients (aged ≥18 years) with type 2 diabetes inadequately controlled with conventional insulin treatment were randomly assigned (1:1:1), via a computer-generated randomisation sequence with an interactive voice-response system, to receive once-weekly dulaglutide 1·5 mg, dulaglutide 0·75 mg, or daily bedtime glargine. Randomisation was stratified by country and metformin use. Participants and study investigators were not masked to treatment allocation, but were unaware of dulaglutide dose assignment. The primary outcome was a change in glycated haemoglobin A1c (HbA1c) from baseline to week 26, with a 0·4% noninferiority margin. Analysis was by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT01191268. Figure 1: Trial profile One patient in the dulaglutide 1・5 mg group and another in the dulaglutide 0・75 mg group died after treatment discontinuation, but while still in the study not taking the study drug. *19 screened patients at one site, eight of whom were randomised, were excluded from analysis because of Good Clinical Practice compliance issues. †Discontinuation of study treatment from week 0 to week 52. One patient in the dulaglutide 1・5 mg group, four patients in the dulaglutide 0・75 mg, and two patients in the glargine group discontinued treatment because of severe, persistent hyperglycaemia (prespecified criteria). Table 1: Baseline characteristics Figure 2: Efficacy variables (A) Least-squares mean change in HbA1c from baseline to weeks 26 and 52 weeks and (B) over time. (C) Proportion of patients achieving HbA1c targets; values above the bars are n (%). (D) Least-squares mean change in FSG from baseline. (E) Baseline and 26 week 8-point SMPG profiles. (F) Change in weight over time. Error bars show 95% CIs. HbA1c=glycated haemoglobin A1c. FSG=fasting serum glucose. SMPG=self-monitored plasma Table 3: Adverse events, changes from baseline in vital signs, and treatmentemergent dulaglutide antidrug antibodies from baseline to 52 weeks Data are n (%), unless otherwise indicated. p values are for dulaglutide vs insulin glargine. Hypersensitivity events were assessed with specifi c standardised MedDRA queries (anaphylactic reaction, angioedema, or severe cutaneous adverse reaction narrow terms). Injection-site reaction was based on a Lilly search category that included specific MedDRA Preferred Terms subsidiary to the MedDRA HLT for injection-site reaction. NA=not applicable. BP=blood pressure. GLP-1=glucagon-like peptide-1. *Reported by at least 0·5% patients across all treatment groups. †The study protocol required that severe hypoglycaemia be reported as a serious adverse event; this requirement might have aff ected the incidence of these categories of reported events. ‡These outcomes were summarised only, no statistical comparisons were done. §Results for both dulaglutide groups combined for all post-baseline observations including follow-up. The appendix shows pancreatic enzyme data. Findings Between Dec 9, 2010, and Sept 21, 2012, we randomly assigned 884 patients to receive dulaglutide 1·5 mg (n=295), dulaglutide 0·75 mg (n=293), or glargine (n=296). At 26 weeks, the adjusted mean change in HbA1c was greater in patients receiving dulaglutide 1·5 mg (−1·64% [95% CI −1·78 to −1·50], −17·93 mmol/mol [−19·44 to −16·42]) and dulaglutide 0·75 mg (−1·59% [−1·73 to −1·45], −17·38 mmol/mol [−18·89 to −15·87]) than in those receiving glargine (−1·41% [−1·55 to −1·27], −15·41 mmol/mol [−16·92 to −13·90]). The adjusted mean difference versus glargine was −0·22% (95% CI −0·38 to −0·07, −2·40 mmol/mol [–4·15 to −0·77]; p=0·005) for dulaglutide 1·5 mg and −0·17% (–0·33 to −0·02, −1·86 mmol/mol [–3·61 to −0·22]; p=0·015) for dulaglutide 0·75 mg. Five (<1%) patients died after randomisation because of septicaemia (n=1 in the dulaglutide 1·5 mg group); pneumonia (n=1 in the dulaglutide 0·75 mg group); cardiogenic shock; ventricular fibrillation; and an unknown cause (n=3 in the glargine group). We recorded serious adverse events in 27 (9%) patients in the dulaglutide 1·5 mg group, 44 (15%) patients in the dulaglutide 0·75 mg group, and 54 (18%) patients in the glargine group. The most frequent adverse events, arising more often with dulaglutide than glargine, were nausea, diarrhoea, and vomiting. Interpretation Dulaglutide in combination with lispro resulted in a significantly greater improvement in glycaemic control than did glargine and represents a new treatment option for patients unable to achieve glycaemic targets with conventional insulin treatment. Funding Eli Lilly and Company. Message 従来治療で管理不十分の2型糖尿病(DM)患者 884人を対象に、インスリンリスプロ併用でのグ ラルギン1日1回投与に対するGLP-1受容体作動薬 dulaglutide週1回投与の非劣性を評価(AWARD-4 試験)。26週時HbA1c平均変化はdulaglutideの 1.5mgおよび0.75mgの両群で非劣性の基準を満た http://www.m3.com/clinical/journal/15492 した。 インスリン業界の老舗のLilly社は持効型インスリンの開発では遅れていた。ジェ ネリックまで出している。しかし、今回の発表で週1回の持効型インスリン相当の 作用がDulaglutideにあることが発表された。ただし、毎日3回の食事用インスリ ンとの併用が必要?なので実臨床でどれだけ受け入れられるか? また、SMBGも朝のみは必要なさそう。貼り付けタイプのSMBGが出るとそちら は解決されそうであるが。 注射回数: GLP1RA+Degludec 2x7 (or 7) vs 今回:1+3x7 /週 1Department of General Internal Medicine, Inselspital, Bern University Hospital, Bern, Switzerland of Medicine and Epidemiology and Biostatistics, University of California, San Francisco 3Service of Endocrinology, Diabetes and Metabolism, University Hospital of Lausanne, Lausanne, Switzerland 4Department of Medicine, University of Minnesota School of Medicine, Minneapolis 5Geriatric Research Education and Clinical Center, VA Medical Center, Minneapolis, Minnesota 6University of Pennsylvania School of Medicine, Philadelphia 7Associate Editor, JAMA 8Department of Physical Therapy, Nicole Wertheim College of Nursing and Health Science, Florida International University, Miami 9Department of Internal Medicine, Erasmus Medical Center, Rotterdam, the Netherlands 10Department of Epidemiology, Erasmus Medical Center, Rotterdam, the Netherlands 11Department of Public Health and General Practice, Norwegian University of Science and Technology, Trondheim, Norway 12Department of Endocrinology, St Olavs Hospital, Trondheim University Hospital, Trondheim, Norway 13Department of Public Health and Primary Care, Leiden University Medical Center, Leiden, the Netherlands 14Department of Public Health and Primary Care, University of Cambridge, Cambridge, United Kingdom 15Radiation Effects Research Foundation, Nagasaki, Japan 16School of Population Health, University of Western Australia, Crawley, WA, Australia 17Department of Medicine, Imperial College London, London, United Kingdom 18Department of Human Metabolism, University of Sheffield, Sheffield, United Kingdom 19Department of Epidemiology and Public Health, University College Cork, Cork, Ireland 20Department of Epidemiology, University of Pittsburgh, Pittsburgh, Pennsylvania 21Cardiovascular Health Research Unit, University of Washington, Seattle 22Levanger Hospital, Nord-Trøndelag Hospital Trust, Levanger, Norway 23Department of Clinical and Experimental Medicine, Geriatric Endocrine Unit, University Hospital of Parma, Parma, Italy 24Institute of Cardiovascular and Medical Sciences, University of Glasgow, United Kingdom 25Department of Public Health, University of Copenhagen, Copenhagen, Denmark 26National Institute on Aging, National Institutes of Health, Baltimore, Maryland 27School of Medicine and Pharmacology, University of Western Australia, Crawley, WA, Australia 28Department of Endocrinology and Diabetes, Sir Charles Gairdner Hospital, Nedlands, WA, Australia 29Institute of Social and Preventive Medicine, University of Bern, Bern, Switzerland 2Departments JAMA. 2015 May 26;313(20):2055-65. doi: 10.1001/jama.2015.5161. Importance Associations between subclinical thyroid dysfunction and fractures are unclear and clinical trials are lacking. Objective To assess the association of subclinical thyroid dysfunction with hip, nonspine, spine, or any fractures. Data Sources and Study Selection The databases of MEDLINE and EMBASE (inception to March 26, 2015) were searched without language restrictions for prospective cohort studies with thyroid function data and subsequent fractures. Data Extraction Individual participant data were obtained from 13 prospective cohorts in the United States, Europe, Australia, and Japan. Levels of thyroid function were defined as euthyroidism (thyroid-stimulating hormone [TSH], 0.454.49 mIU/L), subclinical hyperthyroidism (TSH <0.45 mIU/L), and subclinical hypothyroidism (TSH ≥4.50-19.99 mIU/L) with normal thyroxine concentrations. Main Outcome and MeasuresThe primary outcome was hip fracture. Any fractures, nonspine fractures, and clinical spine fractures were secondary outcomes. Hazard ratios (HRs) were adjusted for age and sex. Data marker sizes are proportional to the inverse of the variance of the HRs. Error bars indicate 95%CIs. Not every outcome was available for each study. Calculations of τ2 were used to measure heterogeneity in effect estimates across cohorts, with a prespecified τ2 (≦0.04) indicating low heterogeneity and greater than 0.04 to 0.36 indicating moderate heterogeneity. All hazard ratios (HRs) were age and sex adjusted. Error bars indicate 95%CIs. The multivariable analysis yielded similar results (eTable 3 in Supplement 1). a The PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) Study was not included because follow-up data were only available for any fracture. b These HRs were adjusted for sex and age as a continuous variable to avoid residual confounding within age strata. c The HUNT (NordTrondelag Health Study), Cardiovascular Health Study, Sheffield, and OPUS (Osteoporosis and Ultrasound Study) studies were not included because follow-up data for any fracture were not available. d The HUNT, Cardiovascular Health Study, Leiden 85Plus, and PROSPER studies were not included because follow-up data for nonspine fractures were not available. e The HUNT, Cardiovascular Health Study, Leiden 85Plus, Sheffield, OPUS, and PROSPER studies were not included because Results Among 70 298 participants, 4092 (5.8%) had subclinical hypothyroidism and 2219 (3.2%) had subclinical hyperthyroidism. During 762 401 person-years of follow-up, hip fracture occurred in 2975 participants (4.6%; 12 studies), any fracture in 2528 participants (9.0%; 8 studies), nonspine fracture in 2018 participants (8.4%; 8 studies), and spine fracture in 296 participants (1.3%; 6 studies). In age- and sexadjusted analyses, the hazard ratio (HR) for subclinical hyperthyroidism vs euthyroidism was 1.36 for hip fracture (95% CI, 1.13-1.64; 146 events in 2082 participants vs 2534 in 56 471); for any fracture, HR was 1.28 (95% CI, 1.06-1.53; 121 events in 888 participants vs 2203 in 25 901); for nonspine fracture, HR was 1.16 (95% CI, 0.95-1.41; 107 events in 946 participants vs 1745 in 21 722); and for spine fracture, HR was 1.51 (95% CI, 0.93-2.45; 17 events in 732 participants vs 255 in 20 328). Lower TSH was associated with higher fracture rates: for TSH of less than 0.10 mIU/L, HR was 1.61 for hip fracture (95% CI, 1.21-2.15; 47 events in 510 participants); for any fracture, HR was 1.98 (95% CI, 1.41-2.78; 44 events in 212 participants); for nonspine fracture, HR was 1.61 (95% CI, 0.96-2.71; 32 events in 185 participants); and for spine fracture, HR was 3.57 (95% CI, 1.88-6.78; 8 events in 162 participants). Risks were similar after adjustment for other fracture risk factors. Endogenous subclinical hyperthyroidism (excluding thyroid medication users) was associated with HRs of 1.52 (95% CI, 1.19-1.93) for hip fracture, 1.42 (95% CI, 1.16-1.74) for any fracture, and 1.74 (95% CI, 1.01-2.99) for spine fracture. No association was found between subclinical hypothyroidism and fracture risk. Conclusions and Relevance Subclinical hyperthyroidism was associated with an increased risk of hip and other fractures, particularly among those with TSH levels of less than 0.10 mIU/L and those with endogenous subclinical hyperthyroidism. Further study is needed to determine whether treating subclinical hyperthyroidism can prevent fractures. Message 潜在性甲状腺機能障害と骨折の関連を、前向き コホート研究13件(参加者約7万人)のメタ解析 で検証。機能正常者に対する潜在性甲状腺機能 亢進症の骨折ハザード比は股関節1.36、全骨折 1.28、非脊椎1.16、脊椎1.51だった。特に甲状 腺刺激ホルモン低値(0.10mIU/L未満)、内因性 潜在性甲状腺亢進症が骨折リスク増加と関連し た。 TSHが0.1を超えていればまぁよいかな? http://www.m3.com/clinical/journal/15495