Nutritional Biochemistry 1
advertisement

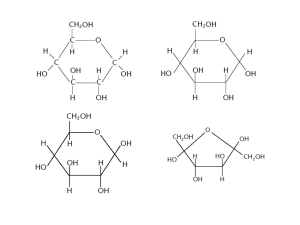
Nutritional Biochemistry 1 CHS 262 CARBOHYDRATES BY Saman Feroze Lecturer Department of clinical nutrition, Applied Medical Sciences King Saud University Introduction INTRODUCTION What is Biochemistry? Biochemistry is the study of the chemistry of life processes. When Biochemistry is linked to diet, health, and disease susceptibility, it comes to be known as nutritional biochemistry. There are mainly 2 classes of molecules: 1. Biological Macromolecules (Lipids, Proteins, Nucleic acids and Carbohydrates) 2. Metabolites (Glucose, glycerol) WHAT ARE CARBOHYDRATES: Carbohydrates (CHO) are single largest component of diet besides water, providing 48% of caloric need. In most CHO, the ratio of hydrogen to oxygen is 2:1. same as water. They are a group of organic compounds whose carbons are extensively hydrated. They can also be defined as aldehyde or ketone derivative of polyhydric alcohols. FUNCTIONS OF CARBOHYDRATES: Main source of energy: body tissues require a constant daily supply of CHO in the form of glucose in all metabolic reactions. CHO exert a protein sparing action. A person must eat a minimum of 100-125gm/day in order to spare the protein. The presence of CHO is necessary for normal fat metabolism. If there is insufficient CHO, large amount of fat is used for energy, which is dangerous due to release of ketone bodies which can cause acidosis which if prolonged can result in brain failure. Glucose is sole source of energy for the brain. Lactose remains in the intestine longer than other dissaccharides and thus encourages the growth of beneficial bacteria. (these bacteria help in synthesis of certain vitamins: B-complex and vit.K) Cellulose and other closely related indigestible CHOs aid in normal excretion. They stimulate the peristaltic movements of the GI tract and absorb water to give bulk to the intestinal contents. CHOs are important components of many compounds such as nucleic acids, connective tissues. CLASSIFICATION: CHO are classified according to number of sugar units: 1. Monosaccharides: simple sugars; they can not be hydrolysed into simpler form and are further classified according to no. of carbon atoms per molecule. a. Trioses: have 3 carbon atoms. Glyceraldehyde and dihydroxyacetone. b. Pentoses: have 5 carbon atoms. Ribose and ribulose. c. Hexoses: Have 6 carbon atoms. Glucose and fructose GLYCERALDEHYDE: Parent compound of CHOs. Formed from the breakdown of hexoses. It exists in two isomeric forms : L and D. D form is naturally occuring. HEXOSES: 1. They all contain 6 carbon, 12 hydrogen and 6 oxygen (C6H12O6). 2. They only differ from each other in the arrangement of hydrogen and oxygen around the carbon atom. 3. They may exist either in aldo or keto form. 4. Nutritionally important hexoses are glucose, galactose, mannose and fructose. 5. They exist both in open chain form or cyclic form (5 membered: Furan or 6 membered: Pyran forms). 6. After cyclization, D-glucose exists in 2 isomeric forms : α-Dglucopyranose and β-D-glucopyranose (called anomers) depending on orientation of hydroxyl group at C1. Diffrerent aldo-CHO with different No. of carbons. Diffrerent keto-CHO with different No. of carbons. •Found free in foods such as honey and fruits. •Major building block of di- and poly saccharide and is transported by blood to all cells where its oxidised for energy. •In blood, conc. Is 70-120 mg/dl. •It is also found in many fruits and honey. •It is also a component of sucrose. •It’s the sweetest tasting monosaccharide. •It is a ketohexose. Ring structures of fructose. Fructose can form both 5 membered and 6 membered ring structures. Galactose: Not found in nature as monosaccharide, but as a part of a disaccharide, lactose. Milk is the prime source of galactose. Mammary glands convert glucose to galactose then synthesize lactose. Infants with Galactosemia lack the enzyme to galactose to glucose which results in toxicity of galactose leading to mental retardation, liver damage and cataracts. Treatment: All forms of milk and lactose be removed from the body. OLIGOSACCHARIDES: CHO built by the linkage of 2 or more monosaccharides by the O-glycosidic bond. DISACCHARIDES: They are composed of 2 monosaccharde units, with same or different. Synthesis C6H12O6 + C6H12O6 C12H22C11 + H2O Hydrolysis/ digestion They account for 35% of dietary carbohydrates. Nutritionally important disaccharides are lactose, sucrose and Maltose. The 2 sugars are joined by an O-glycosidic bond. LACTOSE: (milk sugar) Major dietary CHO for infants. 10% of total CHOs consumed. Lactose is the only natural source of galactose. Lactose is hydrolysed to its monosaccharides by lactase in humans and by β-galactosidase in bacteria. Synthesized also during lactation. Galactose is joined to glucose by a β-1,4-glycosidic linkage. SUCROSE: Table sugar/cane sugar It’s the main transport form of CHOs in plants. Obtained from cane and beet commercially. Made of 2 monosaccharides glucose and fructose which are linked by a glycosidic linkage; α for glucose and β for fructose. It can be cleaved to its 2 components by enzyme sucrase. Provides 20-30% of the total calories. Sucrose----------------------> Glucose + Fructose Sucrase/invertase Glycosidic linkage is α 1-2 bond. MALTOSE: Malt sugar Produced by the hydrolysis of starch and is in turn hydrolysed to glucose by maltase. Present in germinating cereals and not naturally found in diet. Maltose in beer is produced by partial digestion of starch. Glycosidic linkage is α 1-4 bond POLYSACCHARIDES: Composed of many monosaccharides varying from several hundred to over one-half million. Three homopolysaccharides of glucose are starch, cellulose and glycogen; they comprise the major dietary CHOs. Play vital role in energy storage and maintaining the structural integrity of an organism. If all the monosaccharides are the same they are termed homopolysaccharides and if different then they are termed hetropolysaccharides. STARCH: It provides largest proportion of dietary calories and is found only in plants. Its made by plants as principle energy storage form of cereal grains (wheat), and potato. Starch occurs in two forms: Amylose and Amylopectin. Amylose: long straight chain of glucose units, linked through α-1-> 4 glycosidic bonds. It ‘s not soluble in water but forms hydrated micelles, which give a blue color with iodine. Amylopectin: It is a branched chain structure of glucose and contains both α 14 and β 1-6 bond. It gives red violet colour with iodine. Digestive enzymes are specific for the hydrolysis of α 1-4 and β 16 bonds. 1. α -Amylase enzyme [α(1-4) endoglucosidase] hydrolyses only α (14) glycosidic bond present in starch and glycogen. 2. Debranching enzyme [α (1-6) glucosidase[ can hydrolyse only α (1-6) glycosidic bond at the branching point. 3. Cellulase enzyme β (1-4) endoglucosidase can hydrolyse only β (14) glycosidic bonds present in cellulose. Humans do not produce or secrete it. 4. β-amylase enzyme [β(1-4) glucanmalthydrolase] an enzyme found in plants attacks non-reducing ends of amylase to give units of maltose. 5. α -Amylase enzyme and the debranching enzyme work together to break down starch to maltose and glucose. Dextrin: It is another nutritionally important polysaccharide. It’s a more sweet and soluble product resulting from initial breakdown of starch by the α -amylase enzyme when very long glucose chains are split into shorter chains. Dextrin is also formed by the action of dry heat on starch (e.g. toast bread). CELLULOSE: It provides the structural frame work for all plants. It’s the most abundant organic compound in the world. Contains repeated units of glucose linked together by β (1-4) glycosidic bond in a straight chain. Equal quantities of starch and cellulose contain the same caloric values. Calories from cellulose are not available to humans because they don’t produce cellulase enzyme, thus not releasing glucose. Rumen (cattle,sheep) derive calories from cellulose because they have a bacterial enzyme system capable of breaking cellulose linkages. Cellulose provides bulk in the human GI tract and reduces problems of constipation. It absorbs water during transit through the GI tract. GLYCOGEN: also called as animal starch. Structurally very similar to amylopectin, containing both α (1-4) and α (1-6) glycosidic bond. Glycogen is more highly branched and compact as it contains a higher amount of α(1-6) linkages than done amylopectin. After every 8-10 glucose residues, there is a branch containing α(1-6) linkages. The larger amount of branching provides rapid hydrolysis of glycogen to glucose to provide energy. In animals CHO are stored as glycogen , mostly in the liver (2-8%) and muscle (0.5-1.0%). In humans, liver stores 82g and muscle stores 257gm glycogen. After 12-18hr of fasting, the liver becomes completely depleted of glycogen. From muscle it is depleted only after intensive exercise. Muscles can be stored with glycogen again by feeding high CHO diet known as CHO loading. The function of muscle glycogen is to act as an available source of glucose within the muscle. Pyruvate and lactate are the main product of glycogenolysis in muscle. Liver glycogen is mainly concerned with export of glucose units for the maintenance of blood glucose particularly after meals. Very little glycogen is in animal food since most glycogen is converted to lactic acid at the time of slaughtering. Since the amount of glycogen stored in the body at any time is very small, a constant supply of CHO should be always available to the body. Dietary Fibres: The portion of dietary CHO unabsorbed following digestion by human or bacterial enzyme activity are cellulose, hemicellulose, legnin, pectin and gum are usually referred to as fibres. The body can not break them down and so they are not digested by the body and have no nutritional importance. Physiological Importance: The water holding capacity of dietary fibers is very high, so they increase the bulk of stools and facilitate the passage through the colon and help prevent constipation and incidence of colon cancer. They stimulate peristaltic movements of GIT by adding bulk to the intestinal contents. They reduce blood cholestrol level(pectins tend to bind to cholestrol and reduce its absorption and reabsorption from the intestinal tract. Too much fibre depresses the iron utilization fullness. and increases abdominal LACTOSE INTOLERANCE: Many healthy people, adults and children have the inability to digest lactose. It maybe inherited or acquired and results from a deficiency of the enzyme LACTASE. Lactase is necessary to breakdown lactose to galactose and glucose. Under some circumstances, the unhydrolysed sugar passes into the large intestine and gets fermented by the bacteria, inducing a laxative action. Lactase is added in dairy products for fermentation to form yogurt, cottage cheese by changing lactose to lactic acid. BIFIDUS FACTOR: Lactose accounts for 25% of the total dietary CHO. Due to large amount of lactose in mother milk, not all is digested . A small portion is left and fermented in the small intestine to lactic acid. Lactic acid increases the acidity in the lower intestine which promotes the growth of a microorganism, Lactobaccilus bifidus. This L. bifidus helps prevent the growth of other undesirable microorganism. L. Bifidus is found mostly in breast fed infants.