Station 1 - Teacher Pages
advertisement

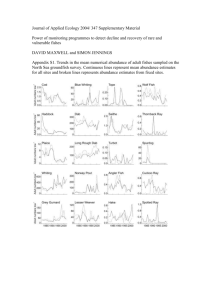
Station 1: Beanium You have in front of you several different bags. Each bag represents the nucleus of the element Beanium. White Beans Neutrons Black Beans Protons 1. Record the data for each bag on the chart. 2. Determine how many isotopes you have. 3. Calculate the percentage of each isotope. This is easy. Count how many of each isotope you have and divide by 10. Then multiple this number by 100. This represents the percent abundance of each isotope in nature. 4. Now determine the mass of each isotope. 5. Use the equation to calculate the average atomic mass of Beanium. 6. Draw the elements symbol, atomic number, and average atomic mass as it would appear on the Periodic Table. 7. Which element is Beanium most similar to on the Periodic Table? Why? Average Atomic Mass: Use the percent abundances and the masses from the table. Atomic mass = % of isotope #1 x (mass isotope #1) + % of isotope #2 of Beanium 100 100 x (mass Isotope #2) + % of isotope #3 x (mass Isotope #3) 100 Station 2: Coinium & Cubium Part 1: 1. Shake 100 pennies in a bag and then dump them out into a cardboard box. A “heads” represents the original isotope of “coinium” and a “tails” represents its decay product. 2. Remove all of the tails so that only the “original nuclides” remain. Record the number of heads that remain in your data table. 3. Put the heads back in the bag, shake, dump, and record the number of heads again. Once again, remove the tails, put the heads back in the bag. 4. Repeat this process until coinium has fully decayed and no heads are thrown. 5. Graph the number of heads (original isotope) as a function of the number of trials. Part 2: 1. Repeat the procedure in Part 1 for cubium. The marked face of the cube is the decay product. Only remove the cubes that land with the marked face up. 2. Graph the number of unmarked sides remaining (original isotope) as a function of the number of trials. Station 3 : Transmutation Dice 1. Roll the dice that contains decay products. Record the decay product in the appropriate place on your table. 2. Roll the dice that contains radioactive particles. Record the radioactive particle(s) in your table. 3. Complete the transmutation equation by determining the missing component. Station 4: Build an Atom pHet Simulation Make sure you are on the Build an Atom Tab. Also make sure the that the simulation is set up as shown below:. 1. Build an atom that has the following components: 3 protons 4 neutrons 3 electrons P N E 2. Use the simulation and your atom to answer the rest of the questions. Station 5: Isotopes and Atomic Mass pHet Simulation Make sure you are on the Mix Isotopes Tab. Also make sure the simulation is set-up as shown below: Station 6: Alpha Decay Start by opening the PhET model “Alpha Decay”. Make sure that you first start by clicking on the single atom tab. It should be setup like the screen below. Station 7: Beta Decay Open the “Beta Decay” PhET model. Make sure that you click on the “Single Atom” tab. It should look like the picture below. Station 8: Nuclear Fission Open the “Nuclear Fission” PhET model. Make sure that you click on the “Fission: One Nucleus” tab. It should look like the picture below. Use this Tab for Questions 1-2. For Questions 3-5, set up as seen below: For Question 6, set up as seen below: Atomic Structure Station Lab Student Sheet Station 1: Beanium 1. Record you data from the bags here! Isotope Name Protons Neutrons Mass Number Number of Bags 2. Average Atomic Mass Calculation: 3. Average Atomic Mass: ________________ 4. Beanium on the Periodic Table (symbol, atomic number, average atomic mass): 5. Which element is Beanium most similar to on the Periodic Table? Why? Percent Abundance Station 2: Coinium & Cubium 1. Fill in the data tables for Coinium & Cubium. Data Table for coinium: Trial # 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 # of heads Data Table for cubium: Trial # # of unmarked faces 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 2. Make a graph for Coinium & Cubium using your data: Coinium Graph Cubium Graph 3. Questions: a. What is the half life of coinium, expressed in number of trials? b. What is the half life of cubium? c. Which decays more rapidly, coinium or cubium? How do you know? Station 3:Transmutation DICE 1. Fill in the table using the dice. Dice Radioactive Particle Dice Radioactive Particle Dice Radioactive Particle Dice Radioactive Particle Dice Radioactive Particle Dice Radioactive Particle Dice Radioactive Particle 2. Which radioactive particle has the highest energy? 3. Which has the lowest penetrating power? What do you need to shield this particle? 4. Which particle is equivalent to an electron? 5. Which particle is equivalent to a helium nucleus? Station 4: Build An Atom pHet Simulation 1. Draw a picture of how you would build your atom below: 2. Circle which element this atom is on this periodic table below: 3. The mass of this atom is: a. 3 mass units b. 4 mass units c. 6 mass units d. 7 mass units e. 11 mass units Explain what ideas you used to choose an answer: ____________________________________________________ ____________________________________________________ 4. The charge of this atom is: a. 0, this is a neutral atom b. -3 c. -1 d. +1 e. +3 5. You start with your atom: 3 protons 4 neutrons 3 electrons 6. You want to change your atom’s properties. Mark YES if a change will work, and mark NO if it will not work. a. Hydrogen, Helium, Lithium, Beryllium, Boron, Carbon are all different elements. If you want to change the type of element your atom is, you can either: (circle) Add a proton or Add a neutron or Add an electron Yes or No Yes or No Yes or No b. If you want to change the charge of your atom, you can either: (circle) Add a proton or Add a neutron or Add an electron Yes or No Yes or No Yes or No c. If you want to change the mass of your atom, you can either: (circle) Add a proton or Add a neutron or Add an electron Yes or No Yes or No Yes or No Station 5: Isotopes & Atomic Mass pHet Simulation 1. Fill in the table using the simulation. Element Isotope #1 P: N: M: P: N: M: P: N: M: P: N: M: % Abundance: % Abundance: % Abundance: % Abundance: Isotope # 2 P: N: M: P: N: M: P: N: M: P: N: M: %Abundance: %Abundance: %Abundance: %Abundance: Isotope # 3 P: N: M: P: N: M: P: N: M: P: N: M: %Abundance: %Abundance: %Abundance: %Abundance: Isotope #4 P: N: M: P: N: M: P: N: M: P: N: M: %Abundance: %Abundance: %Abundance: %Abundance: Average Atomic Mass P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: % Abundance: % Abundance: % Abundance: % Abundance: % Abundance: % Abundance: % Abundance: % Abundance: % Abundance: % Abundance: % Abundance: % Abundance: % Abundance: % Abundance: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: P: N: M: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: %Abundance: 2. Is there a trend to the location of the element and the number of isotopes it has? 3. How do you determine the average atomic mass of an element? 3. Find the average atomic mass if a sample of Cesium, Cs, has the following % abundance: Cs-132 = 20.0%; Cs – 133 = 75.3%; Cs – 134 = 4.7% Station 6: Alpha Decay 1. Observe the decay of Po-211. Write a nuclear equation for the decay of Polonium-211. 2. What has to happen within the nucleus in order for an atom of Polonium-211 to decay? The half-life of Po-211 is approximately 500 ms (half a second). Without using the PhET model, sketch a pie graph indicating the number of undecayed Po-211 atoms for a reaction starting with 100 total atoms. t= 0.5s t=1.0s t=1.5s t=2s Now, simulate the decay of 100 Po-211 atoms by adding 100 atoms from the “Bucket o’ Polonium”. Sketch what the pie graph looks like at the times shown. t= 0.5s t=1.0s t=1.5s t=2s 3. Compare your prediction to the results that you observed. How can you explain any discrepancies? 4. Is it reasonable to assume that if you start with 10 atoms of Polonium, that 0.5s later only 5 will remain undecayed? What if you start with 500 atoms? Explain. Station 7: Beta Decay 1. Observe the beta decay in the PhET model. Write a nuclear equation for the process. 2. When an atom undergoes beta decay, where does the beta particle come from? What other particle is produced in this process? 3. What is the atom mostly made of? 4. What purpose do neutrons serve in the nucleus? 5. Are high or low atomic number elements more likely to undergo beta decay? Look at the examples in the simulations if you need a hint. 6. What radioactive particle is like pure energy? Station 8: Fission Reactions 1. Briefly describe the process by which Uranium-235 can be made unstable. Write a nuclear equation for the process. 2. Suppose that you have 100 atoms of Uranium-235 and you fire a neutron into a single atom. Sketch a qualitative graph of Fissioned U-235 Atoms vs. Time. Using the “Chain Reaction” tab within the model, validate your prediction from question 7. 3. Explain how the PhET model validates/invalidates your prediction made in question 7, citing specific observations. 4. Using the “Chain Reaction” tab, determine the criteria and settings needed to create an atomic bomb. 5. Explain why “weapons-grade” Uranium would not likely contain very much Uranium-238. 6. Use the “Nuclear Reactor” tab to determine the purpose of control rods within a nuclear fission reactor.