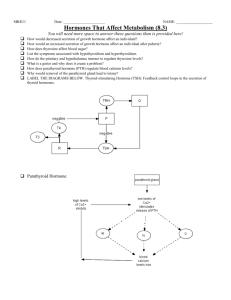
Hormonal regulation
advertisement

Hormone Regulation of Elasmobranch Physiology Chris Bedore and Shannon Long What We’ll Be Covering • Digestion and Energy Metabolism • Growth • Stress • Osmoregulation • Physiological Color Change • Reproduction • Reproduction • Reproduction • Research methods Things to Remember DIGESTION AND ENERGY METABOLISM The Players • Secretin—stimulates secretion of bicarbonate-rich pancreatic juices • Cholescystokinin (CCK)—regulates supply of bile and pancreatic enzymes • Somatostatin (SS)—suppress production of gastric acid, inhibit rectal gland secretion, inhibitory regulation of GH from hypothalamus • Neuropeptide Y (NPY)—promote digestion by increasing blood flow, inhibits gastric acid secretion, pancreatic enzyme release, and gallbladder contraction, inhibit rectal gland secretion • Bombesin/Gastrin-releasing peptide (GRP)— promote digestion by increasing blood flow, effecting acid/enzyme secretion/gut motility, inhibit rectal gland secretion • Vasoactive intestinal polypeptide (VIP)—suppress digestion by reducing blood flow, effecting acid/enzyme secretion/gut motility, stimulates salt excretion by vasodilating the rectal gland and increasing cellular cAMP enzyme, used to regulate water and ion balance • Tachykinins—promote digestion by increasing blood flow, effecting acid/enzyme secretion/gut motility • Insulin—storage/conversion/uptake of energy substrate, pancreas, regulated by nutrient levels, reduction in circulating amino acid levels, no effect on ketones • Glucagon—antagonistic to insulin • Thyroid—alter levels of enzymes in amino acid/lipid metabolism DIGESTION AND ENERGY METABOLISM GROWTH The Players • Growth hormone (GH)—pituitary gland, regulated by GHRH, promotes somatic and skeletal growth • Growth hormone-releasing hormone (GHRH)—regulates GH, stimulatory from hypothalmus • Insulin-like growth factors (IGF-I)—promotes growth of vertebral column STRESS The Players • Chromaffin tissue—masses of neurosecretary cells on kidney, cells secrete epinephrine and norepinephrine, response to acute stress • Catecholamines—epinephrine and norepinephrine, promote mobilization, of energy reserves, increase blood pressure, blood flow to gut reduced, increase oxygen uptake in gills • Hypthalamo-Pituitary-Interrenal axis (HPI axis)— mid-axis lengthwise down body, helps to regulate ions • Corticosteroids—interrenal body, regulated by ACTH, promote nutrient movement through body, inhibit growth and energy storage, aid in retention of sodium • Adrenocorticotropic hormone (ACTH)—regulates corticosteroids, stimulated by CRF, induces hyperglycemia • Corticotrophin-releasing factor (CRF)— hypothalamic compound, stimulates ACTH, regulate interrenal production of 1α-OHB • 1α-Hydroxycorticosterone (1α-OHB)—corticosteroid produced only in elasmobranchs, stimulate retention of sodium and chloride OSMOREGULATION The Players • Renin-angiotensin system (RAS)—used to regulate water and ion balance, series of biochemical steps 1. convert hepatic glycoprotein angiotensinogen to ANG I 2. cleavage to ANG I by ACE makes ANG II • Angiotensin I (ANG I)—inactive form • Renin—enzyme used to convert to ANG I, secreted by juxtanglomerular cells of kidney • Angiotensin converting enzyme (ACE)—promotes cleavage in ANG I to make ANG II • Angiotensin II (ANG II)—biologically active, receptors in interrenal gland/gill/rectal gland/intestine, modulate HPI axis, stimulates 1α-OHB secretion, promotes sodium retention, influence electrolyte balance by reducing GFR and UFR, inhibits salt release from rectal gland, increase drinking rate • C-type natriuretic peptide (CNP)—used to regulate water and ion balance, stimulates production of VIP and rise in salt secretion, expressed in heart and brain, responds to increased cardiac pressure, directly affects rectal gland epithelial cells, binds to NPR-B to increase cGMP and PKC, dilates rectal gland to cause increase in salt release • Natriuretic peptide type-B (NPR-B)—in rectal gland epithelium • Cyclic granosine monophosphate (cGMP) • Protein kinase C (PKC) • Arginine vasotocin (AVT)—regulates osmoregulation, reduces diuresis PHYSIOLOGICAL COLOR CHANGE The Players • Melanocyte—type of chromatophore, contains melanosome which has brown-black melanin • α-Melanocyte-stimulating hormone (αMSH)—regulates physiological color change, produced in neurointermediate lobe of pituitary, when removed- sharks lighten in color, when expressed- sharks darken, controlled by neural signals to hypothalamus • Melatonin—induces skin pallor • Prolactin (PRL)—in par distalis of pituitary, in freshwater ray, regulates physiological color change What Goes On Inside Reproductive Endocrinology- Overview diverse breeding strategies in elasmos therefore, probably diverse regulatory mechanisms this area is largely unknown and mostly hypothetical information is from hormone concentration at a specific time in mating season and deduced from other vert spp. well studied in only a few species (ex: D. sabina, S. tiburo) Reproductive Endocrinology- Overview Brain-Pituitary-Gonad (BPG) axis: primary endocrine regulation, initiated by env. stimuli Reproductive Endocrinology- Overview gonadal steroids: regulate gametogenesis, modulate reproductive behavior, modulate development and function of 2⁰ sex characteristics influence production of GnRH and GTH (neg. feedback) steroid binding sites in hypothalamus potential to alter production of relaxin, calcitonin, thyroid most cycle throughout mating season Reproductive Endocrinology- Anatomy Reproductive Endocrinology- Anatomy GnRH Gonadotropin-releasing hormone: many forms (7?), therefore many functions dfGnRH, sGnRH, cIIGnRH, mGnRH GnRH and GnRH-BPs present in systemic circulation direct action on gonads? different forms present in different parts of the brain Hypothalamus/Forebrain: regulate GTH GnRH Pituitary/Forebrain: regulate pituitary/gonads, convey env. info to initiate BPG GnRH Terminal nerve: regulate repro. processes?! GnRH Midbrain/Hindbrain: regulate sensory sensitivity during reproduction (ie-e-reception)? clasper movement GTH gonadotropin hormone pituitary gland partially regulate steroidogenesis, gametogenesis (systemic GnRH) response to GTH may depend on env. stimuli (H2O temp, photoperiod) and reproductive stage 2 types found so far in elasmos similar structure to FSH, LH in tetrapods? *future research* Female Steroids-Gonadal 3 major gonadal steroids: 17β-estradiol (estrogen)- E2 Progesterone- P4 Testosterone- T Female Steroids-Gonadal Female Steroids-Gonadal E2 2 peaks 1. pre-ovuation/follicle development stimulate vitellogenesis regulate development of oviducal gland 2. late gestation (Squalus acanthias) regulate vitellogenesis regulate secretion of histotroph (Dasyatis sabina) Female Steroids-Gonadal P4 suppress vitellogenesis (opposes E2) viviparous: peaks close to ovulation ↓ late gestation-permits next follicles to develop (S. acanthias) oviparous: regulate timing of oviposition (↑=oviposit) Female Steroids-Gonadal T and DHT (androgens) ↑ during follicle development precursor to E2 synthesis modulate copulatory behavior sperm storage (T) regulate oviposition? *future research- distribution of receptors* Female Steroids-Other Female Steroids-Other Relaxin (Rlx) found in ovary of several species implant and removal experiments↑ cervical area- prep for parturition probably aids pupping and oviposition maintains uterus during gestation (↓ contractions) Sphyrna tiburo- participates in ova release? Female Steroids-Other Thyroid (T3 and T4) interacts with BPG axis, role unknown ↑ during ovulation and gestation (D. sabina) associated with > metabolic costs at this time? S. tiburo highest levels- placental formation Female Steroids-Other Thyroid (T3 and T4) embryo- passed to young through yolk (McComb et al. 2005) regulate rate of development > concentration found in yolk from populations with: larger birth size faster rate of development greater size at maturity higher maternal investment Female Steroids-Other Calcitonin (CT) produced in ultimobranchial gland muscles between pharynx and pericardial cavity ↑ in response to E2 binding on gland maternal gill: ir-cells regulate Ca2+ homeostasis during gestation? mechanism/role unclear Female Steroids-Other Calcitonin (CT) S. tiburo: peaks during yolk dependent stage asstd w/digestion of yolk? ir-cells in duodenum & pancreas of early embyros Female Steroids-Other Calcitonin (CT) D. sabina: peaks during histotroph production no ir-cells in embryo, therefore not involved in embryo nutrition Male Steroids-Gonadal mostly produced in Sertoli cells (in testes) Leydig cells: supplements gonad steroids for regulating stages of spermatogenesis (epididymis and ductus deferens) trends differ among spp, but all regulate aspects of repro. Male Steroids-Gonadal 4 major gonadal steroids: Testosterone (T) Dihydrotestosterone (DHT) Progesterone (P4) 17β-estradiol (E2) Male Steroids-Gonadal Male Steroids-Gonadal T and DHT (androgens) ↑ middle to late spermatogenesis = peak GSI (high # spermatocytes in testes) influence development of spermatogonia and repro. ducts routes of hormone transfer between testes and urogenital system: systemic circulation, binding sites in spermatozoa, enzymes in semen Male Steroids-Gonadal T and DHT (androgens) claspers/cartilage: calcification due to androgens? indirect evidence, but no direct evidence coincides with androgen peak enlarged S. tiburo cephalofoil during male pubertal development but- implant/removal experiments showed no relationship mediated through GH and IGF-1 (after E2 peak)? *future studies* Male Steroids-Gonadal T and DHT (androgens) Male Steroids-Gonadal E2 unsure of role in males- some spp show cycling patterns (D. sabina), irregular variations in others (S. tiburo) D. sabina: receptors in epididymis and seminal vesicles maintain repro. tract function? peak early-middle spermatogenesis regulate early spermatogenesis? *future studies* Male Steroids-Gonadal P4 cycle mirrors androgens (S. tiburo) substrate for androgen synthesis? but- pubertal S. tiburo & D. .sabina: peak precedes androgen peak regulate spermiogenesis and/or spermiation? Male Steroids-Other Relaxin (Rlx) produced by gonads other verts: regulate male fertility S. tiburo: ↑ during late spermatogenesis and copulatory period [relaxinsemen]=1000x [relaxinblood] facilitate insemination through contractability of repro tract of postmated female? Male Steroids-Other Thyroid (T3 and T4) seasonal cycling peak spring and fall aid in ↑ metabolic needs during migration? *future studies* Gonadal Steroid & Development *effect of steroid hormones on development needs more research!* E2, P4, T transferred from female to yolk E2, T probably utilized during development BPG axis activated during maturation steroidogenesis stage specific increases of hormones (S. tiburo), but roles unclear Hormones and Behavior androgens peak in males during copulatory period in some species (ex: D. sabina) may influence certain behaviors- aggression ex: protracted mating period/aggression D. sabina may also affect sensory sensitivities ex: electroreception in D. sabina Dasyatis sabina Tricas et al., 2000 spermatogenesis: production and development of spermatozoa spermiogenesis: last stage; produces mature spermatozoa spermiation: release of spermatozoa from Sertoli cells spermatogonium → spermatocytes → spermatids → spermatozoa Dasyatis sabina Tricas et al., 2000 male steroid hormones: 4 phases: 1. androgen suppression 2. PAI, peak E2, P4 3. androgen decrease 4. SAI Dasyatis sabina phase 1: androgen suppression between mating seasons Dasyatis sabina phase 2: primary androgen increase E2, P4 increases maximum spermatocyte development (max GSI) Dasyatis sabina phase 3: androgen decrease following maximum testis growth and spermatocyte development E2 and P4 also decrease Dasyatis sabina phase 4: secondary androgen increase peak sperm maturation copulatory period Dasyatis sabina Female hormones androgens small, brief peaks winter, spring aggression during mating period? Dasyatis sabina Female hormones E2: 1st peak- maturation of oocytes (March); synthesis and uptake of vitellogenin 2nd peak- late development, parturition transition yolk to histotroph? *future studies* Dasyatis sabina Female hormones P4: 1st peak: near ovulation 2nd peak: parturition Dasyatis sabina androgens, behavior, and protracted mating period: courtship behaviors- males follow females nose to vent chase, bite fins females- enhance mate choice by fleeing Reproductive Endocrinology- Methods Immunocytochemistry, Immunohistochemistry (ICC, IHC): ir-staining of desired hormone ex: GnRH neurons in hypothalamus or terminal nerve Reproductive Endocrinology- Methods ICC, IHC: -collect tissue samples Reproductive Endocrinology- Methods ICC, IHC: - section tissue Reproductive Endocrinology- Methods ICC, IHC: - apply primary antibody, apply secondary antibody with enzyme, provide substrate, then counter-stain Reproductive Endocrinology- Methods Control Electro-Stimulated Female Male P. marinus GnRH neurons (top-female, bottom-male) before (left column) and after (right column) electro-stimulation Reproductive Endocrinology- Methods Radioimmunoassay (RIA) Reproductive Endocrinology- Methods Radioimmunoassay (RIA) uses radiolabeled (ex: 3H) antibody for competitive binding to hormone calculate % bound radiolabel to determine hormone concentration in a sample Reproductive Endocrinology- Methods hormone implants ex: Sisneros and Tricas, 2000 surgically implant hormone tablet/capsule into body wait for it to take effect (usually a # of days) check for changes (RIA, behavior, electrophysiology) Reproductive Endocrinology- Methods hormone implants Reproductive Endocrinology- Methods organ removal (ie pituitary, hypothalamus) similar to hormone implant, but remove organ wait for it to take effect check for changes (RIA, behavioral, electrophysiology) Conclusion/Future Research hormone regulatory mechanisms seem to be similar to other vertebrates many vertebrate hormones first appeared in elasmobranchs MUCH research is needed to determine roles of many hormones. There is less information on elasmo hormones than any other vertebrate group, despite evolutionary role. We need to catch up! Literature Cited Chung-Davidson, Y.-W., M.B. Bryan, J. Teeter, C.N. Bedore, and W. Li. 2007. Neuroendocrine and behavioral responses to weak electric fields in adult sea lampreys (Petromyzon marinus). Hormones and Behavior. (In Review) Gelsleichter, J. 2004. Hormonal Regulation of Elasmobranch Physiology. Pp. 287-324 in Biology of Sharks and Their Relatives (J.C. Carrier, J.A. Musick, and M.R. Heithaus, eds). CRC Press, Boca Raton. Kajiura, S.M., J.P. Tyminski, J.B. Forni, and A.P. Summers. 2005. The sexually dimorphic cephalofoil of bonnethead sharks, Sphyrna tiburo. Biol. Bulletin 209: 1-5 Sisneros, J.A. and T.C. Tricas. 2000. Androgen-induced changes in the response dynamics of ampullary electrosensory primary afferent neurons. J. Neurosci. 20(22): 8586-8595. Tricas, T.C., K.P. Maruska, and L.E.L. Rasmussen. Annual cycles of steroid hormone production, gonad development, and reproductive behavior in the Atlantic stingray. Gen. Comp. Endocrin. 118: 209-225. Trivett, M.K., T.I. Walker, D.L. Macmillan, J.G. Clement, T.J. Martin, and J.A. Danks. 2002. Parathyroid hormone-related protein (PTHrP) production sites in elasmobranchs. J. Anat. 201: 41-52.