Thermodynamics
advertisement

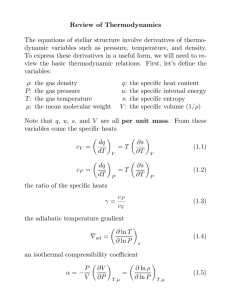
THERMODYNAMIC an introduction Closed and open systems Forms of energy macroscopic microscopic Properties of a system Intensive properties Extensive properties State and equilibrium Zeroth Law 1. Theromodynamics, an engineering approach, 2nd ed., by Yunus A. Çengel & Michael A. Boles, McGraw-Hill, Inc., 1994 2. http://www.wikipremed.com/image _archive.php?code=010304 SYSTEMS AND CONTROL VOLUMES System: System: the thematerial materialinin the theportion portionof of space space to tobe beanalyzed analyzed (closed (closed or oropen) open) Boundary: Boundary: AAseparator, separator,real realor orimaginary, imaginary,between betweensystem systemand and surroundings surroundings (can (canbe befixed fixedor ormovable.) movable.) Surroundings: Surroundings:exterior exteriorenvironment environment U Q, W Mass in Energy in An open system (a control volume) with one inlet and one exit. Mass out Energy out Closed system (Control mass): A fixed amount of mass, and no mass can cross its boundary. Open system (control volume): A properly selected region in space. It usually encloses a device that involves mass flow such as a compressor, turbine, or nozzle. Both mass and energy can cross the boundary of a control volume. Control surface: The boundaries of a control volume. It can be real or imaginary. Forms of energy Fluid Mechanics Combustion Heat Transfer: Conduction, Convection, Radiation Mass Transfer Thermodynamics: The science of energy. The name thermodynamics stems from the Greek words therme (heat) and dynamis (power). First law of thermodynamics— Conservation of energy principle: During an interaction, energy can change from one form to another but the total amount of energy remains constant. Energy cannot be created or destroyed In thermodynamic analysis, it is often helpful to consider the various forms of energy that make up the total energy of a system in two groups: Macroscopic and Microscopic energy The macroscopic forms of energy , are those a system possesses as a whole with respect to some outside reference frame , such as kinetic energy (K.E.)and potential energy (P.E.) Internal energy is defined above as the sum of all the microscopic forms of energy of a system such as : K.E. of the molecules sensible energy, Phase changed latent energy (inter-molecular forces) Bonds in a molecule chemical (or bond) energy (combustion, catalytic electrochemical reaction) Electronic energy Bonds within the nucleus of the atom itself nuclear energy Temperature of the system Fuel Injector Diffuser Compressor Turbine Combustion Chamber Hot exhaust Nozzle Combustion (Fuel+Air) Qin→ H↑ Compressor do Work on air Win→ H↑ Vair, P↑ KE→ H H →Wout Backwork ratio H → KE PROPERTIES OF A SYSTEM Property: Any characteristic of a system. Some familiar properties are pressure P, temperature T, volume V, and mass m. Properties are considered to be either intensive or extensive. Intensive properties: Those that are independent of the mass of a system, such as temperature, pressure, and density. Extensive properties: Those whose values depend on the size— or extent—of the system. Specific properties: Extensive properties per unit mass. Criterion to differentiate intensive and extensive properties. EQUILIBRIUM Zeroth Law of thermodynamic~!! Thermodynamics deals with equilibrium states. Equilibrium: A state of balance. In an equilibrium state there are no unbalanced potentials (or driving forces) within the system. Thermal equilibrium: If the temperature is the same throughout the entire system. Mechanical equilibrium: If there is no change in pressure at any point of the system with time. Phase equilibrium: If a system involves two phases and when the mass of each phase reaches an equilibrium level and stays there. Chemical equilibrium: If the chemical composition of a system does not change with time, that is, no chemical reactions occur. A system at two different states. A closed system reaching thermal equilibrium. TEMPERATURE AND THE ZEROTH LAW OF THERMODYNAMICS The zeroth law of thermodynamics: If two bodies are in thermal equilibrium with a third body, they are also in thermal equilibrium with each other. By replacing the third body with a thermometer, the zeroth law can be restated as two bodies are in thermal equilibrium if both have the same temperature reading even if they are not in contact. Two bodies reaching thermal equilibrium after being brought into contact in an isolated enclosure. Any question ? THERMODYNAMIC The first law The 1st Law States and Processes Work done during volume changed Path between states Cyclic Processes The State Postulate The number of properties required to fix the state of a system is given by the state postulate: ◦ The state of a simple compressible system is completely specified by two independent, intensive properties (P ,T , v ). Simple compressible system: If a system involves no electrical, magnetic, gravitational, motion, and surface tension effects. The state of nitrogen is fixed by two independent, intensive properties. Process Process: Any change that a system undergoes from one equilibrium state to another. Path: The series of states through which a system passes during a process. To describe a process completely, one should specify the initial and final states, as well as the path it follows, and the interactions with the surroundings. Quasistatic or quasi-equilibrium process: When a process proceeds in such a manner that the system remains infinitesimally close to an equilibrium state at all times. Work done during volume changed Work done during volume changed Path between states m m (isometric, isovolumic) Path between states The 1st Law of Thermodynamics Another example of energy transformaiton Qin PE of falling weight KE of paddle Win Heat in water Either heating or stirring can raise T of the water. 1st Law of Thermodynamics : Increase in internal energy = Heat added Work done U Q W dU d Q dW dt dt dt Thermodynamic state variable = variable independent of history. e.g., U, T, P, V, … Not Q, W, … Joule’s apparatus The 1st Law of Thermodynamics 1st Law of Thermodynamics : m(h p.e. k .e.) Q W h u pv(flow work) Path between states :Isothermal Processes Isothermal process : T = constant. V2 W p dV V V1 1 W m R T ln V2 m RT dV m R T ln V V V2 V1 V2 V1 3 3 For U N k T m RT monoatomic gas 2 2 e.g. He Q W m R T ln V2 V1 U 0 Q W Isothermal processes on ideal gas Example : Bubbles ! A scuba diver is 25 m down, where the pressure is 3.5 atm ( 350 kPa ). The air she exhales forms bubbles 8.0 mm in radius. How much work does each bubble do as it arises to the surface, assuming the bubbles remain at 300 K. W n R T ln V2 V1 PV n RT T = const P1 V1 P2 V2 V2 P1 3.5 atm V1 P2 1 atm 3.5 3 4 W n R T ln 3.5 p1 V1 ln 3.5 350, 000 Pa 0.008 m ln 3.5 3 0.94 J Constant-Volume Processes & Specific Heat (Cv) Constant-volume process ( isometric, isochoric, isovolumic ) : V = constant V 0 W p V 0 U Q CV = molar specific heat at constant volume U Q m CV T Ideal gas: U = U(T) CV 1 dQ m dT V isometric processes U ideal gas m CV T Q mCV T for all processes only for const-vol processes Isobaric Processes & Specific Heat (Cp) Isobaric Process : constant P Isotherms W p V2 V1 p V Q U W U p V CP = molar specific heat at constant pressure CP Q mCP T isobaric processes m CP T m CV T p V Ideal gas, isobaric : m CV T m R T CP CV R 1 dQ m dT P Ideal gas Adiabatic Processes No heat lost Q=0 Adiabatic process: Q = 0 (Compression is always a adiabatic process if it is fast enough) Think it in a common sence: Pumping the handle results inU W what? p V const if there is no heat lose (Q=0) T V 1 const adiabat, ideal gas 1. gas pressure increased 2. gas temperature increased p V p1 V1 W 2 2 1 Adiabatic: larger p CP 1 CV Summary: Q/A m m The ideal gas law says p V = n R T, but the adiabatic equation says p V = const. Which is true, (a) the ideal gas law , (b) the adiabatic equation, or (c) both? Explain. mR Implies reversible process no friction and equilibrium process Reversibl work!! mR Diesel Power Fuel ignites in a diesel engine from the heat of compression (no spark plug needed). Compression is fast enough to be adiabatic. If the ignite temperature is 500C, what compression ratio Vmax / Vmin is needed?? Given : Air’s specific heat ratio is = 1.4, & before the compression the air is at 20 C. T V 1 const 1 / 1 Vmax Tmin Vmin Tmax 1 / 1.4 1 273 K 500 K 273 K 20 K 11 Q/A : Name the basic thermodynamic process involved when each of the following is done to a piston-cylinder system containing ideal gas, tell also whether T, p, V, & U increase or decrease. (a) the piston is lock in place & a flame is applied to the bottom of the cylinder, (b) the cylinder is completely insulated & the piston is pushed downward, (c) the piston is exposed to atmospheric pressure & is free to move, while the cylinder is cooled by placing it on a block of ice. (a) isometric; T , p , (b) adiabatic ; T , p , V =const, U . V, (c) isobaric ; T , p =const, V , U . U . Cyclic Processes Cyclic Process : system returns to same thermodynamic state periodically. 。 A four-process cycle Example : Finding the Work done in a cycle An ideal gas with = 1.4 occupies 4.0 L at 300 K & 100 kPa pressure. It’s compressed adiabatically to ¼ of original volume, then cooled at constant V back to 300 K, & finally allowed to expand isothermally to its original V. How much work is done on the gas? AB (adiabatic): WAB WAB BC (isometric): CA (isothermal): work done by gas: pA VA pB VB 1 V pB p A A VB 1 100 kPa 4.0 L 1 41.41 pA VA VA 1 741 J 1 VB 1.4 1 WBC 0 WCA n R T ln VA VC pA VA ln 4 555 J WABCA WAB WBC WCA 186 J From 1st law of thermodynamic, We know that: “You cannot build a perpetual motion ! Since sou cannot get more energy out than you put in(conservation of energy).” But…… About the efficiency: Can we know how much work done at least we can get after putting energy into the machine? About the direction of heat: When you’re holding a cup of coffee , Why doesn’t your hand get colder as the coffee become hotter and hotter , It does not against with the 1st law! The 1st law is not enough to explain both questions~! We are going to the world of 2nd law Any question ? THERMODYNAMIC The second law The 2nd Law Clasusius statements Kelvin-Planck statements Limits on performance Irreversible Carnot cycle Entropy statement We’ll miss you, Qc … (Clausius statement) no process is possible where the sole result is the removal of heat from a low-temp reservoir and the absorption of an equal amount of heat by a high temp reservoir (Kelvin-Planck) no process is possible in which heat is removed from a single reservoir w/ equiv amount of work produced Rudolf Clausius (1828-1888) Lord Kelvin (1824-1907) Max Planck (1858-1947) Heat Engine Efficiency Limits on performance An irreversible processes normally include one or more of the following processes : 1. 2. 3. 4. 5. 6. 7. 8. Heat transfer through a finite temperature difference Unrestrained expansion of a gas or liquid to a lower pressure Spontaneous chemical reactions Spontaneous mixing of matter at different compositions or states Friction-sliding friction as well as friction in the flowing fluids Electric current flow through a resistance Magnetization or polarization with hystersis Inelastic deformation Limits on performance Reversible cycle Carnot Cycle Nicolas Léonard Sadi Carnot 1796-1832 A Carnot Cycle consists of four steps: Isothermal expansion (in contact with the heat reservoir) Adiabatic expansion (after the heat reservoir is removed) Isothermal compression (in contact with the cold reservoir) Adiabatic compression (after the cold reservoir is removed) Every processes in the cycle are reversible! How about its efficiency ~! Efficiency of a Carnot cycle Since no one can create a 0 k cold reservoir or a ∞ k heat reservoir . Carnot efficiency is a theoretical maximum and it can’t reach 100% Entropy T v.s. S diagram of Carnot cycle The 2nd law of thermodynamic S 0 If a process occurs in an isolated (closed and adiabatic) system the entropy of the system increases for irreversible process and remains constant for reversible processes. IT NEVER DECREASES…. Any question ? Cycles A diagram can be drawn with any pair of properties ◦ P-T ◦ P-V (allows the net work of a cycle to be determined: W=integral of pdV ◦ T-S (gives the net heat of a cycle; recall 2nd law which states: dsdQ/T -> Q=integral of Tds! If you can convert some of the heat to work, you have an engine! Cycle Types Premixed Charge – Otto Cycle, gasoline, spark-ignition engine Non-premixed charge or stratified charge engine (compression ignition or Deisel cycles) Gas Turbines – Brayton Cycle Other cycles: Rankine, … Where to start: Air (ideal gas) cycles Assume no changes in gas properties (cp, MW, , …) due to changes in composition, temp., …called the IDEAL air cycle! •REAL cycles must consider fuel-air mixture which is compressed, burned, expanded,… with accompanying changes in thermodynamic properties Premixed Charge – Otto Cycle How can we take that into calculation? We need to simplify it ! Premixed Charge – Otto Cycle Expand 4 P s v Burn: Constant Volume Simplify 5 3 s 1 2, 6 Compress Process v Blowdown V (cylinder volume) Description Assumption Mass in Other info cylinder 1 -> 2 Intake P = const Inc. 2 -> 3 Compress s = const Const 3 -> 4 Burn v = const Const 4 -> 5 Expand s = const Const 5 -> 6 Blowdown v = const Dec. 6 -> 1 Exhaust P = const Dec. 1. Intake valve open 1. Exhaust valve closed 2. intake valve closed 3. spark fires 5. exhaust valve opens – pressure “blows down” Otto Cycle P Heat Added 4 s v T 5 4 v Expansion s 3 s 1 v 2, 6 3 Compression V 1,2,6 s 5 v Heat Rejected S Thermal efficiency hth=what you get/what you pay for hth Work out Work in Heat in hth - cv(T5 T4) cv(T3 T2) - T5 T4 T3 T2) cv(T4 T3) T4 T3 Adiabatic reversible compression/ expansion T3 V3 T2 V 2 ( 1) thus and T4 V4 T5 V 5 V4 V3 1 V5 V2 rc ( 1) where rc: compression ratio Thermal efficiency • After some algebra: h 1 1 th,otto rc independent of heat input efficiency increases as rc increases ◦ why not go to rc -> why not? ◦ geometrical limitations, heat loss, irreversibilities (high compression -> high T -> high heat loss), knock Thermal efficiency h 1 1 th rc • Example: Auto engine: rc~8; ~1.3 hth~0.46 (theoretical); hth~0.30 at best (expt) Differences: • • • • • Heat Loss to valves, cylinder walls Incomplete combustion Friction Blow by, valves leak Throttling (Pexhaust Pintake) Diesel Cycle Combustion 2 3 P Compression 6 Stratified charge engine - fuel injected after air compressed Expansion - heat release doesn’t occur instantly since fuel will take more time to burn than in the premixed case. 4 This is bec. fuel must mix, vaporize, than burn. Takes time. - To model this, combustion process 1,5 assumed to occur at increasing V volume, constant pressure New ratio V3/V2 introduced Diesel Cycle V V2 Define: β 3 depends on the heat input can show: h 1 th,diesel 1 β 1 β 1 rc >1 for b>1 thus: h h th,diesel th,otto and: h h th,diesel th,otto when b=1 Ideal Brayton Cycle (Gas Turbines) 1. 2. 3. 4. Isentropic Compression (1->2) Constant pressure heat addition (2->3) Isentropic expansion (3->4) Constant pressure heat rejection (4->1) P 2 Combustor . m 2 3 4 1 Wc Compressor 3 Wnet 4 Turbine 1 V Ideal Brayton Cycle Heat Added P 2 3 T 3 Expansion s v 2 4 1 Compression 1 V s 4 v Heat Rejected s Ideal Brayton Cycle cp T T Wc m 1 2 cp T T Wt m 4 3 cp T T Qin m 3 2 cp T T Qout m 1 4 ( ) / W 1 h net 1 where PR= PR th Q in Note: cp T T T T m 4 2 1 3 Wnet = Wt – Wc = 1 P 2 P 1 T2 T3 T1 T4 P 2 P 1 Tsunami &Quake-induced nuclear power plant crisis—six nuclear reactors at Fukushima Daiichi What do we learn from this catastrophe?