化学驱油技术相关进展
advertisement

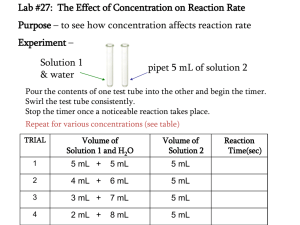
EXPERIMENT 1 Surface tension determination with the maximum differential pressure method Wang Zengbao 2012.11 1. Experimental purposes (1)Master the principles and methods of maximum differential pressure method for the determination of surface tension. (2)Measure the surface tension of n-butyl alcohol solution, and understand the concept of surface tension and influencing factors. (3)Learn Gibbs equation and its applications. 2. Experimental principles Polyacrylamide can be synthesized from acrylamide triggered by ammonium persulfate: (NH4)2S2O8 nCH2 CH CONH2 [CH2 CH]n CONH2 Polyacrylamide can hydrolyze in alkaline solution, which generates partially hydrolyzed polyacrylamide: [CH2 CH]n + yH2O + zNaOH CONH2 [CH2 CH]x [CH2 CH]y CONH2 COOH [CH2 CH]z + (y + z)NH3 COONa 2. Experimental principles 2 P R P R P1 P2 1 2 R 2 2 p2 r 2 1 p1 r p2 2 1 (1) p1 P —The additional pressure which is formed when the water drops down in the wide mouth bottle —The surface tension R —The curvature radius of (N/m) the bubble 2. Experimental principles 2 P R 2 2 p2 r 2 1 p1 r p2 2 1 (1) p1 Therefore, at the same temperature, once the ΔP1, ΔP2 are measured, and the IFT of the liquid(water) whose IFT is known is found out according to the temperature table,then we can calculate the IFT of the unknown liquid from the formula 。 3.Apparatus and reagents Apparatus One set of device to measure the IFT with the method of maximum differential pressure,Bottle, rubber pipette bulb. Reagents N-butyl alcohol (analytically pure), distilled water. 4.Experimental procedures (1)Wash the external case and the capillary tube of the instrument used to measure surface tension with lotion. First add a little lotion into the external case,rotation it inclined so that the lotion can contact with the external case(be careful not to let the lotion outflow from the side of the tube).Then insert the capillary tube and maintain the inclined external case still.Rotate the capillary tube to make the lotion contact with the capillary tube.Adsorb some lotion with the rubber pipette bulb to the capillary tube to wash the inside of the capillary tube.Pull the spent lotion to its original bottle and wash the external case and the capillary tube with running water fully.Finally,wash the external case and the capillary tube with distilled water three times respectively.Then we can go on with the following experiences. 4.Experimental procedures (2)Put some distilled water into the external case(regard as liquid of known IFT. See Appendix II to get its surface tension).Insert the capillary tube into the external case and plug the plug tightly and let the tip of the capillary tube touch the liquid surface exactly.Read out the zero level h0 of the tube beneath the inclined tube manometer(At this point both ends of the inclined tube manometer connect with the atmosphere).Install the device according to figure21,where the separatory funnel is filled with tap water.. 4.Experimental procedures (3)Open the piston of the separatory funnel to let the water in it drip slowly into the wild-mouth bottle.Then the pressure inside the bottle increase gradually and bubbles will pass through the end of the capillary tube. Read out the highest level h1 from the inclined tube manometer when the first bubble pass through it.Repeat the same procedures three times and calculate the average value h2. 4.Experimental procedures (4)Drain the distilled after the maximum differential pressure has been measured.Wash the external case and the capillary tube once with butanol with the concentration of 0.002mol/L.Then add the solution and measure its maximum differential pressure just like the distilled water.Measure the maximum differential pressure of the butanol solution with the concentration of 0.05,0.10,0.15,0.20,0.25,0.30,0.35mol/L . (Note: every time the solution is changed, we should use the Pending liquid to wash the external case and the capillary tube) (5) Record the experimental temperature. 5.Experimental results processing (1)Isolate the surface tension of distilled water at experimental temperature from the appendix. (2)Calculate the surface tension of solution with different concentration of normal butyl alcohol by p 2 2 p1 2 (3)Using surface tension as vertical ordinate,concentration as horizontal scale,draw the б-c figure of nomal butyl alcohol solution on the coordinate paper. (4)Select some points from theб-c figure and draw the tangents of different concentration curve .Then calculate the surface adsorptive capacity according to the Gibbs equation ;and draw the isothermal adsorption curve of nomal butyl alcohol solution 6.Questions (1)During experiment ,if the capillary insert the liquid surface by 1mm ,how much error will bring about? (2)During experiment ,why do we slow the barbotage ? (3)During experiment,why do we measure the surface tension of different concentration solution from from low to high. EXPERIMENT 3 Preparation and electrophoresis of colloids Wang Zengbao 2011.4 1. Experiment purposes (1)Learn the basic principles of preparation of sol (colloid), and master the main methods of preparation sol; (2)Mensurate the electrokinetic potential of AgI (silver iodide) sol by measuring the movement of interface. 2. Experiment principles Colloidal sol is a highly dispersed system with solid of very small solubility dispersing in liquid. Its diameter changes in the range of 10-7 to 10-9 meter. The formation of stable colloidal material involves two facets: the dimension of the dispersed phase within the colloidal range; the particles dispersed in liquid should not aggregate, so stabilizing agent usually added. Basically, the preparations of colloidal systems involve either degradation of bulk matter or aggregation of small molecules, ions or particles. 2. Experimental principles Electrophoresis of colloids Under an external electric field the colloidal particles moving to the positive electrode or negative electrode, this phenomena is called electrophoresis. Electrophoretic potential can be measured by electrophoresis through two methods: microscopic method and macroscopic method. 3.Apparatus and reagents Apparatus Electrophoresis apparatus; Electrophoresis tube; Stopwatch; Pt electrode, 2; 100mL Beaker, 3; Plastic head dropper ,2;25mL Graduated flask, 2 , and so on. Reagents Silver nitrate solution (0.01mol/L); Potassium iodide solution (0.01mol/L); Potassium chloride solution (0.005mol/L) 4.Experiment procedures Preparation of AgI negative solution 20mL 0.01mol/L KI (Potassium iodide) solution is added to a 100mL beaker, 18.7mL 0.01mol/L AgNO3 (Silver nitrate ) solution is dropwise added to the beaker under stirring, AgI negative colloid is prepared. 4.Experiment procedures Determination of potential (1) The electrophoresis apparatus should be washed clean (2) Fixed the electrophoresis apparatus vertically on the iron support stand (3)Close the piston, Add colloid through the funnel with plastic head dropper (4) Assistant solution is added into the U-tube (5) Open the piston slowly, when colloid up to 0-tick,close the piston (6)Gently insert two electrodes, immerge assistant solution 1cm (7)Push the electrophoresis apparatus’s “start” button, and record the time that the interface rising 0.5, 1.0, 1.5 cm cost with a stopwatch. (8)Turn off the power, measured the distance of two electrodes by a ruler. Wash the apparatus 5.Experiment results processing (1)Summarize the method of preparation of colloid; (2)Calculate ξ potential of AgI negative colloid. 6.Questions (1)What are the similarities and differences among different preparation methods? (2)Why must the conductivity of the assistant solution be equal to that of the colloid? What is the function of that for calculating the potential? (3)What are the reasons that cause the color, definition and moving speed differences between the rising interface and declining interface? (4)What are removed through dialysis of Fe (OH)3? Is there any way to detect degree of purification? Is it to remove all the ions dispersed in the solution during dialysis? EXPERIMENT 4 Coagulation of Inorganic Electrolyte and Flocculation of High Molecule Polymer Geng Jie 2012.11 1. Experiment purposes (1) Master principle and method of sol coagulation. (2) Verify coagulation symbol and valence number rule of electrolyte. (3) Understand the flocculation of the watersoluble polymer to the sol. 2. Experiment principles (1) Coagulation of inorganic electrolyte The entire process that the sol losses coagulation stability and then losses dynamic stability is called coagulation. The sol can be caused to coagulate by electrolyte. The reason is that the electrolyte can decrease ξ potential of sol, and the higher electrolyte concentration, the lowerξpotential is. When theξpotential drops to a certain value, the sol will lose coagulation stability, and then will occur coagulation. The higher ion valence number of opposite sign, the greater coagulation ability of electrolyte is. This is consistent with SchlZe--Hardy Rules. M+:M2+:M3+=(25~150)∶(0.5~2)∶(0.01~0.1) 2. Experimental principles (2) Mutual coagulation phenomena The mutual coagulation phenomenon is that mixing the two sols with the opposite electric charge can also cause coagulation. There are usually two mechanisms. Electric nature neutralization about two kinds of sol particles of opposite electric charge. A sol with high counter-ion of opposite electric charge sol. 2. Experimental principles (3) Flocculation of high polymer When the concentration of polymer is very low, main manifestation of polymer is flocculation for sol. Flocculation is created due to "bridging" of polymer to sol particles. "Bridging" theory: When the concentration of polymer is very low, polymer chains can be absorbed on several colloidal particles at the same time. Several particles join together through "bridging". Due to rotation and vibration of polymer chains, colloidal particles join together and subside. 3. Instruments and Chemicals Instruments 722 spectrophotometer, 100mL conical flask, 10mL micro-burette, 5mL pipette, 10mL pipette, 10mL test tube, 20mL test tube, 50mL plug graduated cylinder, 50mL beaker, 100mL beaker. Chemicals 0.01mol/L KCl, 0.001mol/L K2SO4, 0.001mol/L K3(COO)3C3H4OH, Fe(OH)3 sol,clay sol. 4.Experiment procedures (1) Coagulation of electrolyte to sol In the three clean, dry 100mL conical flasks, add 10mL Fe (OH)3 sol with pipette respectively. Then instill electrolyte solution listed in Table 4-1 with micro-burette respectively, and add each drop with fully oscillation. Sol does not appear roily at least one minute before adding the second. Record the volume of electrolyte solution when just appear roily, and list in Table 4-1. 4.Experiment procedures Table4-1 coagulation record about different electrolytes to sol Fe(OH)3 sol electrolyte KCl K2SO4 K3(COO)3 C3H4OH concentration of volume of all electrolyte coagulation value electrolyte solution(mol/L) solution(mL) (mmol/L) 4.Experiment procedures (2) Mutual coagulation between clay sol and Fe(OH)3 sol Take six dry test tubes, and add the amount of Fe (OH)3 sol according to Table 4-2 in each test tube. Then add the clay sol in all test tubes, so that the total volume of the sol is 6mL in each test tube. Shake each test tube, rest 10 minutes, and record the coagulation phenomena of each test tube. 4.Experiment procedures Table4-2 the record sheet about mutual coagulation of sols test tube number 1 2 3 4 5 6 Fe(OH)3 sol 0.1 0.5 1 3 5 5.5 clay sol 5.9 5.5 5 3 1 0.5 coagulation phenomena 4.Experiment procedures (3) Flocculation of high polymer Take three 50mL plug graduated cylinder with similar internal diameter, respectively add 30mL clay sol with pipette, respectively add the 0.02% partially hydrolyzed polyacrylamide (HPAM) solution of 2×106 molecular weight 2drops﹑10drops and 40drops and fro turn 10 times, rest 2 minutes, draw 5mL solution at 2cm department under the surface, with 722 spectrophotometer (the Appendix eight), at the wavelength of 420nm, measure optical density with the distilled water as blank, and fill the data into Table 4-3. 4.Experiment procedures Table4-3 the record sheet of flocculation about HPAM to clay sol clay sol HPAM 0.02% 30 mL 30 mL 30 mL 30 mL 0 2d 10d 40d C% system concentration of HPAM (%) T (T=I/I0) D (D=-LogT) Ar (Ar=D/D0) *Ar is the ratio of sol optical density with HPAM and without HPAM. If Ar=1, that is not complete flocculation and if Ar = 0, that is complete flocculation. So Ar can show the degree of flocculation. 4.Experiment procedures (3) Flocculation of high polymer In this study, the volume of HPAM added is not fixed, only for reference. Because the best flocculated concentration change with the molecular weight of HPAM, degree of hydrolysis, the sol concentration and preparative conditions. Therefore, the amount of HPAM added can make appropriate changes based on the actual situation. 5.Experiment results processing (1) Particularly observe various phenomena in experiment, record these phenomena and data and fill in the form with the data. (2) According to the results, determine the electric nature of Fe (OH)3 sol and clay sol. (3) Compare the coagulation values of different electrolytes, and verify SchlZe–Hardy law. (4) Draw up Ar-c curve with the quality percentage of HPAM as X-axis and flocculation efficiency Ar as Y-axis, and explain it.. 6.Questions (1) Why must the Fe (OH)3 sol go through dialysis before flocculation test? (2) Whether is the coagulation values of different electrolysis to the same sol same? Why? (3) When the concentration of polymer is higher in the sol, what will arise, and why? EXPERIMENT 5 Preparation identification and breakdown of emulsion Wang Zengbao 2012.12 1. Experimental purposes (1) Preparation of different types of emulsion; (2) Understand some method of the emulsion preparation; (3) Familiar with some damage emulsion method. 2. Experimental principles Emulsion is a liquid dispersed in another immiscible liquid in the form of dispersion. There are two types of emulsion, namely oil in water type (O/W) and oil-water type (W/O). Only two immiscible liquids can not form a stable emulsion. To form a stable emulsion, emulsifier must exist. In general emulsifiers are mostly surface active agent. Surfactant could reduce the surface energy and form protective film of the droplet surface, or make the droplet surface charged to stabilize the droplet emulsion. 2. Experimental principles Emulsifiers could also be divided into oil in water emulsifier and oil-water emulsifier. Generally monovalent metal fatty acid soap (eg sodium oleate) is hydrophilic than hydrophobic, so it’s oil in water emulsifier. Divalent or trivalent fatty acid soap (such as magnesium oleic acid) is lipophilic than hydrophilic, so is the water-in-oil emulsifier. The following three methods could identify the two types of emulsions: (1)Dilution (2)Conductivity method (3)Staining 2. Experimental principles (1) Dilution Add one drop of emulsion in the water, if it spreads out immediately, it means that the dispersion medium is water, so it is oil in water emulsion; And it is water in oil emulsion if it is not immediately disperse. 2. Experimental principles (2) Conductivity method Water phase generally contains ions, so its conductivity is much greater than the oil phase. When the water medium is dispersion medium (continuous phase), the conductivity of emulsion is large; the other hand, if oil is continuous phase and water is the dispersed phase, water droplets are not continuous, so the conductivity of emulsion is weak. Two electrodes which are connected to DC power supply and ammeter are inserted into the emulsion solution. If there is significant deflection of ammeter, the emulsion is oil in water solution; if the pointer does not move, the emulsion is water in oil emulsion. 2. Experimental principles (3) Staining The dye which is only soluble in oil but does not dissolve in water or only water-soluble (such as Sudan Ⅲ which is red dye and only soluble in oil but not water-soluble) is added into the emulsion. If the dye dissolved in the dispersed phase, the droplets stained one by one appears in the emulsion. If the dye is soluble in the continuous phase, the emulsion showed uniform dye colors inside. The type of emulsion could be determined by the dispersion of dye. 2. Experimental principles Methods of destroy the emulsion (1) Adding the demulsifier (2) Adding electrolyte (3) Heating (4) Electrical method 2. Experimental principles (1)Adding the demulsifier Demulsifier is often anti-emulsifier. For example, the water in oil emulsion made by adding magnesium oleic acid could be broken by adding sodium oleate. The sodium oleate which is hydrophilic could adsorb on the liquid surface and form the hydration shell that could reduce the emulsification of magnesium oleic acid and break the emulsion. If adding excess sodium oleic acid, the water in oil emulsion may change into oil in water emulsion. 2. Experimental principles (2) Adding electrolyte Different electrolytes may have different effects. In general, by adding electrolytes into oil-in-water emulsion, it could change HLB of the emulsion and reduce the stability of the emulsion. . 2. Experimental principles (3) Heating Elevated temperature could reduce the emulsion agent adsorption on the interface and thin the solvent layer and low the medium viscosity and enhance the Brownian motion. Therefore, reducing the stability of emulsion helps emulsion damage. (4) Electrical method Under the action of the high voltage field, droplet could deform and connect to each other, then decreased dispersion results in the destruction of the emulsion . 3. Equipment and medicine Equipment 100mL conical flask with a plug 2, Large test tube 5, 25mL graduated cylinder 2, 100mL beaker 3, Small dropper 3, constant current source 1, milliammeter 1, one pair of electrodes Medicine Benzene (chemical pure), Sodium oleate (chemical pure), 3mol/L HCl solution, 1%, 5% sodium oleate solution, 2% magnesium oleic acid benzene solution , 0.25mol/LMgCl2 aqueous solution, saturated NaCl solution, Sudan Ⅲ solution. 4.Experimental procedures (1) Preparation of emulsion Add 15mL 1% aqueous solution of sodium oleate into 100mL conical flask with a plug. Then add 15mL of benzene (each of adding about 1mL) with severe shaking after each adding of benzene until there is no benzene layer phase. Type Ⅰemulsion is obtained. 15mL 2% benzene solution of sodium oleate is added into another 100mL conical flask with a plug. Then 15mL of water is added (each of about plus 1mL) with severe shaking after each add of water until there is no water layer phase. Type Ⅱ emulsion is obtained. 4.Experimental procedures (3) Destruction and phase conversion of the emulsion ①Take two clean test tube, add 1 ~ 2mL typeⅠ and type Ⅱ emulsion to each test tube, dropwise add 3mol/L HCl solution, then observe the phenomenon. ②Take two clean test tube, add 1 ~ 2mL type Ⅰ and type Ⅱ emulsion to each test tube, heat the tubes in water bath, then observe the phenomenon. 4.Experimental procedures (3) Destruction and phase conversion of the emulsion ③Take two clean test tube, add 2~3mL typeⅠ and type Ⅱ emulsion to each test tube, dropwise add 0.25mol/L MgCl2 solution with severe shaking after each add of MgCl2 solution, then observe the destruction and phase conversion of the emulsion. (Identify the phase conversion using dilution method, the same below) 4.Experimental procedures (3) Destruction and phase conversion of the emulsion ④Take two clean test tube, add 2~ 3mL typeⅠ and type Ⅱ emulsion to each test tube, dropwise add saturated NaCl solution with severe shaking after each add, then observe the destruction and phase conversion of the emulsion. ⑤Take two clean test tube, add 2~ 3mL typeⅠ and type Ⅱ emulsion to each test tube, dropwise add 5% sodium oleic acid solution with severe shaking after each add, then observe the destruction and phase conversion of the emulsion. 5.Experimental results processing Record and collate the phenomena observed in experiments, and analyze causes. 6.Questions 1. What is common point for the various emulsion identification methods? 2. It is said that if the water is more than oil it could form oil in water emulsion, whereas the water-oil. Is it right or wrong? Illustrate it by trial results. 3. Could the phase conversion method be used as demulsification? Could the demulsification method be used as phase conversion? 4. Could the two immiscible liquids form emulsion automatically by adding emulsifier? EXPERIMENT 6 Synthesis and hydrolysis of polyacrylamide Wang Zengbao 2011.4 1. Experimental purposes 1.Be familiar with addition polymerization by the synthesis of polyacrylamide from acrylamide. 2. Be familiar with the hydrolysis of polyacrylamide in alkaline solution. 2. Experimental principles Polyacrylamide can be synthesized from acrylamide triggered by ammonium persulfate: (NH4)2S2O8 nCH2 CH CONH2 [CH2 CH]n CONH2 Polyacrylamide can hydrolyze in alkaline solution, which generates partially hydrolyzed polyacrylamide: [CH2 CH]n + yH2O + zNaOH CONH2 [CH2 CH]x [CH2 CH]y CONH2 COOH [CH2 CH]z + (y + z)NH3 COONa 3.Apparatus and reagents Apparatus Constant temperature water bath, beaker, graduated cylinder, stirring rod, electronic balance. Reagents acrylamide (chemical pure), ammonium persulfate (analytical pure), sodium hydroxide (analytical pure) 4.Experimental procedures 1. The addition polymerization of acrylamide (1) Weigh the mass of beaker and stirring rod with a electronic balance (this mass would be used later). Then add 2g acrylamide and 18mL water in the beaker to match a 10% acrylamide solution. (2) In the constant temperature water bath, heat the 10% acrylamide solution to 60 ℃. Then add 15 drops of 10% ammonium persulfate solution to trigger the addition polymerization of acrylamide. (3) In the process of addition polymerization, keep stirring slowly and observe the changes of solution viscosity. (4) Stop heating half an hour later, and the product is polyacrylamide. 4.Experimental procedures 2. Polyacrylamide hydrolysis (1) Weigh the obtained polyacrylamide, calculate the needed water to make 5% polyacrylamide solution. (2) Add the required water in polyacrylamide solution, then stir it with a stirring rod and observe the dissolution of the polymer. (3)Weigh 20g 5% polyacrylamide solution ( the rest is control group) , add 2mL 10% sodium hydroxide, then place it in a boiling water bath and heat it to above 90 ℃ for hydrolyzation. (4)In the hydrolysis process, stir it slowly and observe the viscosity changes, check the ammonia release (with wet pH paper of wide range). (5)The beaker is removed from the boiling water bath half an hour later, and the product is partially hydrolyzed polyacrylamide. (6) Weigh the product mass, add the loss of evaporated water, and 5% partially hydrolyzed polyacrylamide is made. Then compared the solution viscosity before and after hydrolyzation. (7)Pour the prepared polyacrylamide into the recycling bottle. 5.Experimental results processing Explain all kinds of observed phenomena in the experiment. 6.Questions 1. What is the impact of ammonium persulfate amount on the molecular weight of synthesized polyacrylamide? 2. Why the temperature is raised to 60 ℃during the synthesis of polyacrylamide? 3. Analyze the factors that affect the molecular weight of polyacrylamide. EXPERIMENT 7 Determination of Polymer Molecular Weight by Viscometric Method Wang Zengbao 2011.4 1. Experimental purposes Learn and understand one method to determine the polymer molecular weight . 2. Experimental principles The polymer molecular weight is an average value owing to the polydisperse molar mass distribution. The polymer molecular weight determined by viscometric method is called the Viscosity-average molecular weight ( M v). Viscometric method includes multi-point method and onepoint method,we use one-point method. 2. Experimental principles Because At 0 t ln r ln ln 0 t0 and 0 r SP 0 0 Use these formulas to get Mv 0 t t0 SP 0 t0 1 ( SP ln r ) 2c [ ] k M v Mv 2. Experimental principles In this experiment, for measurement of the viscosity of polymer solution at different concentration, the Ubbelohde-type viscometer illustrated in this Fig. 1, 2, 3—Branch pipe; 5, 8, 9-Glass bulb; 4, 6-Scale; 7-Capillary We need record the time between the scale 4 and 6 3.Apparatus and reagents Apparatus The Ubbelohde-type viscometer, stopwatch, suction bulb,graduated flask, Glass Constant temperature water bath Reagents Polyacrylamide (PAM, industrial products), Sodium nitrate (NaNO3, AR), Distilled water. 4.Experimental procedures (1) Place the viscometer vertically in a thermostat bath maintained at 30 ℃, and let it stand for about 15min to attain the specified temperature. (2) Add 15mL 1 mol/L sodium nitrate solution through branch pipe 3 into the glass bulb 9 . Close tube 1 with a finger and pull the sample solution up to scale 4 by gentle suction from the top of tube 2, and stop the suction. Remove the finger from tube 1 and immediately close the end of tube 2. Confirming that the meniscus of liquid column is cut off at the scale 4, open the end of tube 2 to make the sample solution flow down through the capillary tube 7. Record the time required for the sample solution to fall from the upper scale (scale 4) to the lower scale (scale 6) by stopwatch. Repeat the above measurements at least 2 times and take the average value . 4.Experimental procedures (3) Cleaning of the Ubbelohde viscometer three times with 0.01g/100mL polyacrylamide solution. (4) Determine the polyacrylamide solution flow time to fall from the scale 4 to the scale 6 using the method mentioned above. 5.Experimental results processing Calculation the SP 0 t t0 0 t0 ln r ln t ln 0 t0 Mv 1 ( SP ln r ) 2c [ ] k M v Mv 6.Questions 1. Summarize the experimental methods for determining molecular weight and their scope of application. 2. Why is NaNO3 added to determine of polymer molecular weight by viscometric method? Are other salts reasonable? 3. How to choose the concentration of polymer solution in the multi-point method?