Benzene - WordPress.com
advertisement
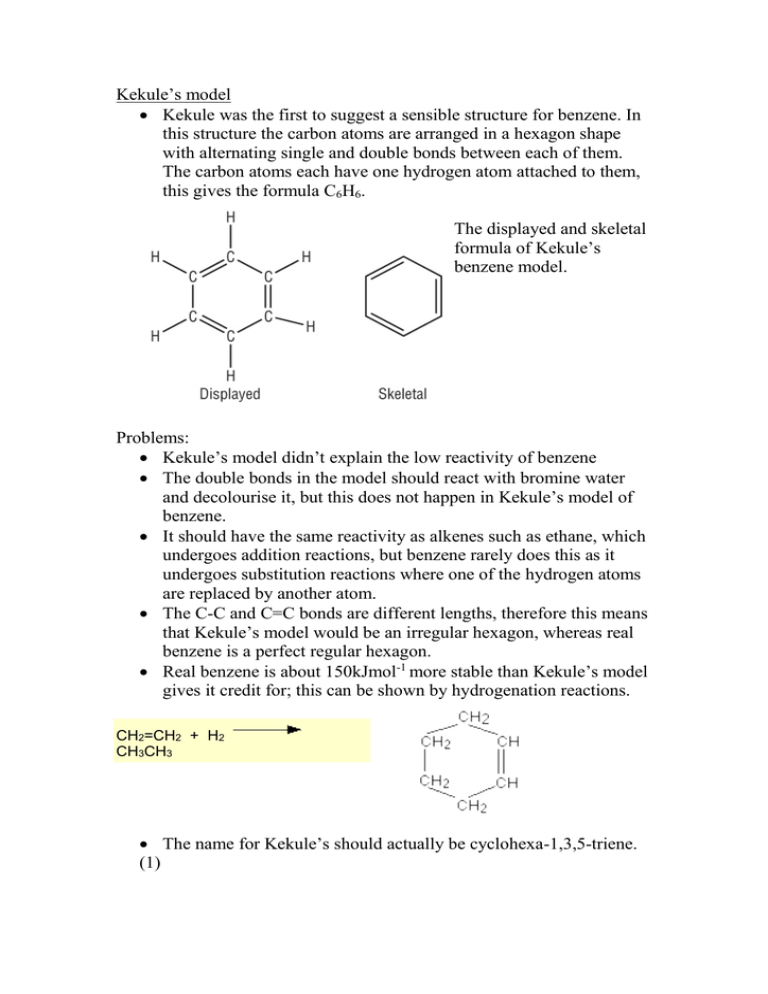
Kekule’s model Kekule was the first to suggest a sensible structure for benzene. In this structure the carbon atoms are arranged in a hexagon shape with alternating single and double bonds between each of them. The carbon atoms each have one hydrogen atom attached to them, this gives the formula C6H6. The displayed and skeletal formula of Kekule’s benzene model. Problems: Kekule’s model didn’t explain the low reactivity of benzene The double bonds in the model should react with bromine water and decolourise it, but this does not happen in Kekule’s model of benzene. It should have the same reactivity as alkenes such as ethane, which undergoes addition reactions, but benzene rarely does this as it undergoes substitution reactions where one of the hydrogen atoms are replaced by another atom. The C-C and C=C bonds are different lengths, therefore this means that Kekule’s model would be an irregular hexagon, whereas real benzene is a perfect regular hexagon. Real benzene is about 150kJmol-1 more stable than Kekule’s model gives it credit for; this can be shown by hydrogenation reactions. CH2=CH2 + H2 CH3CH3 The name for Kekule’s should actually be cyclohexa-1,3,5-triene. (1) The current model Benzene was discovered in 1825 by the English scientist Michael Faraday. In 1834 the German chemist Eilhardt Mitscherlich heated benzoic acid with lime and produced benzene. Benzene is in a cyclic arrangement of 6 carbon atoms with alternating single and double bonds. Modern bonding models explain the structure and stability of benzene in terms of delocalisation of six of its electrons. Delocalisation in this case refers to the attraction of an electron by all six carbons of the ring instead of just one or two of them. This delocalisation causes the electrons to be more strongly held, making benzene more stable and less reactive than expected for an unsaturated hydrocarbon. Benzene is known to have a planar structure and bond angles of 120 degrees. It has a boiling point of 80.1 degrees Celsius and a melting point of 5.5 degrees Celsius. (2) The current model of benzene (showing the overlap of p orbitals). References 1. Chem Guide Bonding In Benzene http://www.chemguide.co.uk/basicorg/bonding/benzene1.html 2. Carey, Francis A. Benzene http://www.britannica.com/science/benzene







