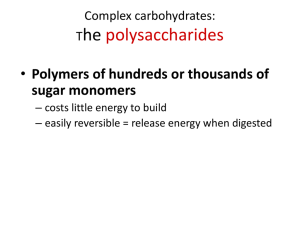
polymer of glucose
advertisement

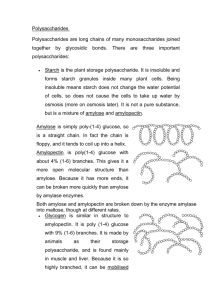
Carbohydrates- IB Biology Essential Idea- Compounds of carbon, hydrogen and oxygen are used to supply and store energy. Molecular Models Kits • In your groups, you have a kit. We will be making models with these over the next several class periods. • To begin, you will receive a handout with the models to be made each day. I will instruct you on which ones to build as we go through each period. Please stay on task and do only the ones required. You must get a stamp in order to receive credit. No exceptions. Review: Polymer Principles •Four classes of macromolecules: –Carbohydrates –Lipids –Proteins –Nucleic Acids •Polymers are made up of smaller parts called monomers. •Polymers are formed through condensation reactions. •Polymers are broken apart through a hydrolysis reaction. General Information about Carbohydrates •Carbohydrates are composed of C, H, and O CH2O (CH2O)x C6H12O6 –“Carbo”-contains carbon –“Hydrate”- Compound containing chemically combined water. •Often end in “-ose” Is this a carbohydrate? Is this a Carbohydrate? Is this a carbohydrate? Is this a Carbohydrate? Carbohydrate Functions –Immediate energy source for cells –Energy storage for later use –Raw material for building other molecules –Important role in cell membrane recognition About 17 KJ of energy per dry gram. About the same as protein, but ½ that of lipids Monosaccharides -one sugar unit (monomer)– are the simplest carbohydrates •Backbone of 3-7 carbon atoms •Form ring structures in cells •Characterized by sweet taste •Have several polar -OH groups, so they are soluble in water. (The many –OH groups can hydrogen bond with water molecules) Carbons are numbered On your lab paper, draw Glucose Number the carbons On your glucose, circle all the hydroxyl (–OH) groups Label the slight positive and slight negatives on the highlighted O’s and H’s. HINT: Remember polarity and unequal pull of electrons Using the molecular model kits, build a model of glucose Notice orientation of hydroxyl (OH) groups Initial when complete Glucose • C6H12O6 • A product of photosynthesis • Main energy source for cells- used in ATP synthesis during cellular respiration Draw a water molecule hydrogen bonding off of EACH highlighted H and EACH highlighted O. –Be sure you correctly orient the H’s and O’s of the water molecule –Show the hydrogen bond with a dotted line (you should end up drawing 10 H2O’s) Some example monosaccharides • • • • Glucose Ribose Deoxyribose Fructose Deoxyribose and ribose are the building blocks for nucleic acids. Found in DNA Found in RNA** Fructose • Found in fruits • Used by plants to attract animals to the fruit for seed dispersal. Isomers • Glucose and fructose have the same chemical formula C6H12O6 but different structural arrangement of the atoms (called isomers) The monosaccharides glucose, fructose, and galactose are isomers. • They contain the same atoms but in different arrangements All are C6H12O6 Disaccharides • Di = 2 • Saccharide = sugar Disaccharides are formed in Condensation Reactions http://kisdwebs.katyisd.org/campuses/MRHS/teacherweb/hallk/Teacher%20Docu ments/AP%20Biology%20Materials/Chemistry%20of%20Life/Condensation%20 and%20Hydrolysis%20Reactions/conde_shell.html 2.3.1 Monosaccharide monomers are linked together by condensation reactions to form disaccharides and polysaccharide polymers. Disaccharide #1 Gosh! Isn’t it sweet?! The two glucose molecules are holding hands. Maltose: C12H22O11 (glucose + glucose – H2O) is a dimer of glucose. It provides energy for germinating seeds. Maltose – between which carbon #’s are the glucoses connected? H2O Using the molecular model kits: BUILD MALTOSE Notice orientation of hydroxyl (-OH) groups. When finished with maltose • Combine each group’s maltose’s to make one long chain. Draw this. This is a model of starch. Sucrose (glucose + fructose) is a transport form of sugar used by plants and harvested by humans for food and used as table sugar. Lactose (galactose and glucose) is present in milk Splenda: A modified disaccharide • Splenda is just a modified form of sucrose • Notice the chloride ions that replace the hydroxyl groups Other sweeteners Steviol Complex Carbohydrates: The Polysaccharides •Polymers of hundreds or thousands of sugar monomers –costs little energy to build –easily reversible = release energy when digested Storage Polysaccharides • What does it mean to store something? Storage Polysaccharides –Starch (polymer of glucose) •Found in PLANTS •Formed in roots and seeds as a form of glucose storage –Glycogen (polymer of glucose) •Found in ANIMALS •Formed in the liver and muscles as a form of glucose storage Structural Polysaccharides •Cellulose (polymer of glucose) –Most abundant organic compound on Earth –Found in the cell walls of plants –Indigestible for most animals due to orientation of bonds between glucoses Structural Polysaccharides •Chitin (polymer of modified glucose units) –Found in the outer coverings of insects, crabs, and spiders –Found in the cell walls of many fungi Starch vs. Cellulose Starch: •Polymer of a-glucose •Highly branched •Has a 1-4 linkages •Used for storage in plants. Cellulose •Polymer of b-glucose •Linear, does not branch •Has b 1-4 linkages •Most animals lack the enzyme to break the b 14 linkages (so we can’t digest it). Digesting starch vs. cellulose starch easy to digest enzyme cellulose hard to digest enzyme Cow can digest cellulose well; no need to eat other sugars Gorilla can’t digest cellulose well; must add another sugar source, like fruit to diet Helpful bacteria • How can herbivores digest cellulose so well? – BACTERIA live in their digestive systems & help digest cellulose-rich (grass) meals Ruminants ITell eatme about the rabbits, WHAT! again, George! Summary of Carbohydrates • Monosaccharides: Glucose, Fructose, Galactose, Ribose • Disaccharides: Lactose, Maltose, Sucrose • Polysaccharides: Starch, Cellulose, Glycogen, Chitin – Alpha (starch) & Beta (cellulose) linkages – Chitin: exoskeleton, suture, and fungi (cell walls) Starch Cut-Out Activity 1. Get in your lab groups, at each table 2. Each person pick up one color of the glucose molecules, and 2 water drops. 3. Cut out the glucoses on the lines, and make them even. 4. Attach the two glucoses with tape. 5. Write H, and OH on the water drops. 6. Affix the water below the O. 7. Attach all the glucoses at your table, with a water in between each. Then connect the entire class.