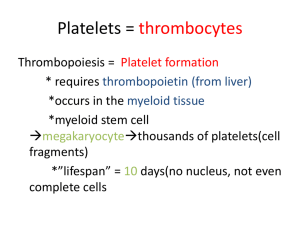
Bare Bones Blood and Bone Marrow
advertisement

Hemostasis/Coagulation Gregory S. Travlos, DVM, DACVP National Institute of Environmental Health Sciences Research Triangle Park, NC 27709 919-541-0653 Travlos@niehs.nih.gov Hemostasis The process by which bleeding is arrested. • It is a series of physiological and biochemical events which terminate in the formation of an insoluble fibrin clot Hemostatic Sequence: • Interaction between vessel wall and platelets • Blood coagulation • Fibrinolysis Hemostatic Component Interactions Thompson & Harker, 1983 Blood Vessels Intact endothelium forms a thromboresistant surface • Required for the free flow of blood; does not promote platelet adherence or activate coagulation • Passive mechanisms: • Endothelial glycocalyx (negative charge - repels like-charged particles, e.g.,platelets). • Presence of a2-macroglobulin at cell surface (protease inhibitor). • Active mechanisms: • Endothelial cells remove platelet aggregation promoters from circulation (e.g., PGF1, bradykinin, serotonin, adenine nucleotides). • Secretion of PGI2 - potent inhibitor of platelet aggregation, induces vasodilation. Proteoglycan matrix of the vessel wall influences thrombogenicity. • Heparin, heparan sulfate and dermatan sulfate have anticoagulant activity; other glycosaminoglycans and hyaluronic acid do not. • Veins have the highest concentration. Endothelium Besides their role in thromboresistance, endothelial cells have additional synthetic functions. • Produce Von Willebrand’s factor • Absorbed by platelets; needed for adherence to collagen • Produce plasminogen activator (tPA) • Mediates fibinolysis • Injured cells release thromboplastin (factor III) • Activates the “extrinsic” coagulation cascade • Others (e.g., type III and IV collagens, elastin, fibronectin, etc.) Blood Vessel Structure Thompson & Harker, 1983 Platelets Adhere to exposed collagen (platelet plug) • Occurs in seconds; can control hemorrhage of minute injuries Secretory functions; mediators of coagulation and fibrinolysis • Releases ADP; sticky and promotes platelet adherence • ADP activates phospholipase A2 which stimulates thromboxane A2 synthesis • Release of membrane fibrinogen, factor V, factor VIII and calcium • Release of membrane platelet phospholipid. Platelet - TEM microtublules OCS granules mitochodrion Ultrastructural and Functional Platelet Anatomy Platelets - cont. The role of platelets in hemostasis is as important as the coagulation mechanism. • Thrombocytopenia, thrombasthenia or thromobopathia - impair hemostasis • Thrombocytosis or thrombocythemia - may impair, but usually promotes clotting (predisposes to thrombosis). Platelets promote hemostasis by: • Release of ADP and other agonists; promotes adherence. • ADP activates phospholipase A2 which stimulates thromboxane A2 synthesis • Thromboxane A2 - stimulates vasoconstriction and platelet aggregation • Release of membrane fibrinogen, factor V, factor VIII and calcium • Components of coagulation localized at site of injury • Release of membrane platelet phospholipid. • Accelerates the “intrinsic” and “common”pathways of coagulation Prostaglandin Metabolism Harlan & Harker, 1981 Hemostatic Platelet Functions Thompson & Harker, 1983 Platelet Response When a vessel is injured or severed a brief, local, reflex vasoconstriction occurs. • Reduces blood flow at site. • Maintained by vasoactive compounds (platelets, surrounding tissues). Passing platelets adhere to exposed collagen. • Occurs in seconds; initially adhere in a single layer and become activated. • Severe injury - collagen serves as a potent platelet activator. • Less severe injury - vWF and fibrinogen become the major activators. The adhered platelets undergo a conformational change. • From discoid to development of long filopodia. • Activation of GP receptors for fibrinogen and/or vWF (GPIIb/IIIa and GPIb/IX/V). Structure of the GPIb-IX-V receptor Tablin, 2000 Platelet Response to Agonists Platelets - unstimulated Characteristic discoid shape SEM plates; Gentry, 2000 Addition of ADP Addition of thrombin (mild stimulation) (strong stimulation ) Shape change (elongation and crescents) and filaform process formation (arrows) Increased spreading, filaform process extension (arrows) and aggregate formation (stars) Platelet Response cont. Activated platelets release their a-granule and dense body contents inducing additional platelet recruitment. • Dense granules - ADP, serotonin and epinephrine. • alpha-granules - fibrinogen (and vWF in human and pig). • Synthesis and release of PAF and TxA2. The agonists accelerate the development of an irreversible platelet aggregate (platelet plug). • Reversible v. irreversible responses. • Thrombocytes of birds and reptiles do not respond to ADP. • Serotonin and epinephrine: • Serotonin - shape change (rat, g. pig and dog); aggregation (human, rabbit, cow, horse, pig, sheep and cat). • Epinephrine - only human, primate, cat and horse platelets appear responsive. • Either serotonin or epinephrine combined with another agonist - strong response in all species. Platelet Response cont. More about agonists. • Platelet Activating Factor (PAF). • Cow, horse, sheep, primate, dog, g. pig and rabbit respond to PAF. • Human less sensitive and rat and mouse are insensitive to this agonist. • Thromboxane A2 (TxA2). • Strong agonist - human, g. pig and rabbit. • Weak agonist - horse. • Insensitive - rat, cow, pig. In real life, however, platelets are exposed to multiple agonists from platelets and other cells (e.g., red cells, ADP; white cells, PAF). Platelet Aggregation to Thrombin Harlan & Harker, 1981 Hemostatic Plug Formation Baumgartner & Muggli, 1980 Coagulation System Consists of a cascading system of proteins • Primarily originating from liver (except factor III) • Circulate in inactive form (except, possibly, factor VII) • System includes: • • • • • Enzymatic factors Non-enzymatic factors Tissue thromboplastin (factor III) Calcium (factor IV) Platelet phospholipid (PF 3) - structural component; accelerates factor activation • Anticoagulant factors The coagulation system consists of three pathways (intrinsic, extrinsic and common) Procoagulant Factors Coagulation Systems - cont. Enzymatic factors • Circulate as non-active zymogens - must be activated to function • Activated enzymatic factors are not consumed during clotting (except factors II and XIII) • Partial deficiency results in partial loss of clotting ability • Activated enzymatic factors inhibited by antithrombin III (complexed with heparin) and some alpha-2-glycoproteins • Enzymatic factors: • XI and XII (contact factors) • II, VII, IX and X (vitamin K-dependent factors) • XIII (clot stabilizing factor or fibrin-stabilizing factor) Coagulation Systems - cont. Non-enzymatic factors • Originate from liver but associate with platelet membranes (also found in plasma) • Normal clotting with partial deficiency; almost total absence needed to affect hemostasis or clotting • Clotting consumes these factors - absent in serum • No known natural inhibitors • Considered reactive proteins - increased during inflammatory and neoplastic processes (except factor III) • Non-enzymatic factors: • Fibrinogen (factor I) • Factor V • Factor VIII:C (associated with Von Willebrand’s factor) Coagulation Cascade Interactions Does this turkey have factor XII? Of course, he does But, his feathered companion does not Coagulation Systems - cont. Clot stabilization • • • • • • Fibrin stabilizing factor (factor XIII) forms fibrin strand cross-links. Synthesized by monocytes and hepatocytes. Zymogen is activated by thrombin (plus calcium). A very small amount of factor XIII (2 - 10%) is adequate for hemostasis. Converts soluble fibrin monomers (unstable) to a fibrin polymer (stable). Lead, silver, zinc and snake venoms are known inhibitors. Coagulation Inhibitors The activity of coagulation system must be attenuated. • Numerous inhibitors are found in blood. Coagulation is controlled by three types of actions. • Inhibition of converting enzymes (e.g., AT III, C1 esterase inhibitor, a2macroglobulin, a2-antiplasmin, a1-antitrypsin, HC-II). • Act on one or more of the converting enzymes (activated factors). • AT III-heparin pathway: major system - 80% of the thrombin inhibitory action in plasma. • Destruction of protein cofactors (e.g., TM-PC-PS system). • TM-PC-PS system degrades cofactors V & VIII:C, inhibiting prothrombinase and tenase complexes, respectively. • Blocking receptor availability needed for complex formation (e.g., Tissue factor pathway inhibitor (TFPI) and annexin V). Proposed Mechanism of AT III-Heparin System Lysine sites Serine site AT III Arginine site Thrombin Antithrombin III Th Heparin H AT III Th H Proposed Mechanism of Thrombomodulin, Protein C and Protein S (TM-PC-PS) System F-Xa Prothrombin Activated platelet PS Ca++ F-Va x Thrombin Ca++ Activated Protein C Thrombin Thrombomodulin Protein C Proposed Mechanism of Tissue Factor Pathway Inhibitor (TFPI) Activity F-Xa F-Xa TFPI TFPI F-Xa TFPI F-VIIa Tissue factor Endothelium Anticoagulant Factors Fibrinolytic System Method for removing clots and maintenance of a patent vascular system and fibrin deposited during inflammation and tissue injury must be removed. • Plasmin (serine protease) primarily responsible for fibrinolysis. • Produced in the liver and kidney, it circulates in an inactive form (plasminogen). • Activators: tissue plasminogen activator (tPA), cytokinases-urokinases (urine, CSF, tears, saliva, milk, bile, synovial, prostatic and amniotic fluids), erythrocyte erythrokinase, neutropil activator and factor XII-dependent activator (XII-prekallikrien-hageman factor cofactor complex). • In addition to fibrin and fibrinogen, plasmin will hydrolyse a variety of proteins. • While plasminogen is normally found in blood and body fluids, plasmin is usually absent due to numerous antiplasmins. • Inactivators: antithrombin III, a2-macroglobulin, a1-antitrypsin and C1 inactivator. Fibrinolytic System and Factors Regulating Fibrinolysis (Fibrinogenolysis) Activation Damaged endothelium Plasminogen Inhibition Kallikrein Plasminogen activator inhibitor e-aminocaproic acid FHIIa Prekallikrein tPA Streptokinase Urokinase Plasmin a2-Antiplasmin Biodegradation of FV, FVIII, FIX, FXI fibrinogen Firbrinogen/fibrin a2-Macroglobulin Complement activation Fibrin/fibrinogen Degradation products Degradation of Fibrin/Fibrinogen Fibrinogen or Fibrin Plasmin Fragment X Small Peptides Plasmin Fragment Y Fragment D Small Peptides Plasmin Fragment E Fragment D Small Peptides Evaluation of Hemostasis Fundamental physiology and pathophysiology of hemostasis is similar in mammalian species. • Variables identical for laboratory animals and human patients Platelets • Platelet count - detection of thrombocytopenia • Clot retraction - non-anticoagulated blood • Failure to separate - platelet function defect or thrombocytopenia • Bleeding time (BT)- in vivo test; simple; low sensitivity • • • • Used to evaluate platelet function defects Thrombocytopenia - prolongs BT Clotting factor deficiency does not alter BT Vascular disease (eg., scurvy) can prolong BT (humans, guinea pigs) Considerations for Blood Collection Clean/smooth surfaces • Want to avoid platelet clumping or activation of factor XII • Use plastic or siliconized glass for sample collection • Animal blood clots faster than human blood - prime needle with anticoagulant Collect sample from an endothelial-lined vessel and careful venipuncture • Want avoid contamination with tissue juice (factor III) • Small clot activates coagulation system invalidating results • Samples from indwelling catheters are usually unacceptable Sample Handling/Anticoagulants Plasma samples separated from cells within 30 minutes • Perform analyses immediately • Plasma samples may be quickly frozen (dry ice/alcohol or liquid nitrogen) and stored at -70o for analysis at a later date • Activity of factors V and VIII is lost rapidly in samples held at room temperature Citrate (trisodium salt) is the anticoagulant of choice. • Oxalate anticoagulants are acceptable - not commonly used • Heparin - unacceptable • EDTA - unacceptable (except for indirect evaluation of fibrinogen concentration by heat precipitation and refractometry) Evaluation -cont. Activated Coagulation Time (ACT) - in vivo test • Measures (seconds) time to clot formation in fresh whole blood • Careful attention to sample collection/handling • Platelet counts <10,000 cause slight increase in ACT • Results from lack of platelet phospholipid for test • Increased ACT suggests factor deficiency in intrinsic or common pathways • Deficiency must be 5% of normal to prolong ACT Activated Partial Thromboplastin Time (APTT) • Measures (seconds) time to clot formation in citrated plasma • Increased APTT - factor deficiency in intrinsic or common pathways • • • • Deficiency must be 30% of normal to prolong APTT Fibrinogen <50 mg/dL will prolong APTT; inflammation may shorten APTT Sensitivity increased with saline-diluted plasma Heparin therapy prolongs APTT - differentiate using a 1:1 dilution with normal plasma Evaluation -cont. One-Stage Prothrombin Time (OSPT, PT) • Measures (seconds) time to clot formation in citrated plasma • Rabbit or synthetic tissue thromboplastin preferred; human origin reagent gives longer PT times • Increased PT - factor deficiency in factor VII or common pathway • Deficiency must be 30% of normal to prolong PT • Fibrinogen <50 mg/dL will prolong PT • Sensitivity increased with saline-diluted plasma Russel’s Viper Venom Time (RVVT) • Measures (seconds) time to clot formation in citrated plasma • Increased RVVT - in or common pathway but insensitive to factor VII deficiency • Deficiency must be 30% of normal to prolong RVVT • Fibrinogen <50 mg/dL will prolong RVVT • Sensitivity increased with saline-diluted plasma Evaluation -cont. Thrombin Clotting Time (TCT) • Measures (seconds) time to clot formation in citrated plasma • Increased TCT - decreased fibrinogen concentration (<100 mg/dL), dysfibrinogenemia, increased FDP concentration, heparin therapy Fibrinogen Concentration (factor I) • In most species, fibrinogen is 100 - 400 mg/dL • Fibrinogen decreases in DIC, severe liver insufficiency and hereditary hypofibrinogenemia • Inflammation can increase fibrinogen concentration Evaluation -cont. Fibrin-Fibrinogen Degradation Products (FDP) • Measures, by latex agglutination, the concentration of products of fibrinolysis; D-dimer assay is another method for measuring FDP • Increased FDP - occurs with disseminated intravascular coagulation or severe internal bleeding • In most species, normal FDP is <10 micrograms/mL Example Acute oral study in dogs Animals given 3 X LD50 in food • Brodifacoum • Bromadiolone • Diphacinone Coagulation studies • ACT • RVVT • PT