Physics Study Guide: Accuracy, Precision, Significant Digits
advertisement

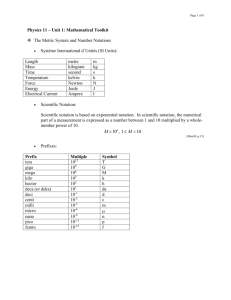
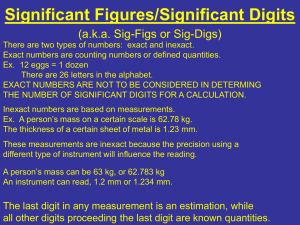
Name: _____________________________ Period: ______ Chapter 1 Study 1 Guide accuracy physics precision significant digits 1. The study of matter and energy is ________physics____________________. 2. The valid digits in a measurement are called the __significant digits_________. 3. ________Accuracy________________________ describes how well the results of a measurement agree with the real value. 4. The degree of exactness of a measurement is called _______precision_________________________. 5. A device with very small divisions on its scale can measure with a. scientific notation c. agreement b. precision d. fundamental units 6. An atomic mass unit is measured at 1.660 x 10-27kg, a number that has _____ significant digits. a. 1 c. 3 b. 2 d. 4 Use the graph on the right to answer the following questions. 7. Which variable is the independent variable? _____time__________________ 8. Which is the dependent variable? _____distance_______________ 9. Explain why the slope at 2.0 s is greater than the slope at 1.0 s. _the ball is falling a greater distance at 2.0 s than at 1.0 s. __ ___________________________ ___________________________ 10. About how far does the ball fall in 1.8 s? ___16 m____________________ 11. Why do physicists work in SI units? SI (metric) units are based on powers of 10 so it is easy to convert between units. ____________________________________________________________ _________________________________________________________________ 12. What does the term resolution mean when referring to measurement? Resolution refers to the smallest measurement a device can make. The smaller the resolution, the more precise the device. _____________________ _________________________________________________________________ 13. What are significant digits? Is zero a significant digit? Use examples to explain your answers. Significan digits are the valid digits in a measurement and depend on the precision of the device used to make the measurement. Zeroes can be significant if they are sandwiched or precision zeroes. ___________________ 14. What is the difference between accuracy and precision? Use examples to explain your answers. Accuracy is obtaining the correct or actual measurement and precision is the degree of exactness of the measurement (the more decimal places in a measurement the more precise) ______________________________________ 15. What are the advantages of using graphs? Give an example. Graphs display data visually. For example, speeds can easily be compared with position-time graphs. __________________________________________ _________________________________________________________________ 16. The base SI unit for length is the ___________. a. foot b. meter c. inch d. kilogram 17. The metric prefix that means 1x106 is ___________. a. pico c. mega b. nano d. giga 18. How many significant digits are in the measurement 2.560 x 104? a. 1 c. 3 b. 2 d. 4 Name: _____________________________ Period: ______ Chapter 1 Study 3 Guide 19. Which of the following is a more precise measurement—the length of a tabletop measured with a stick calibrated in centimeters as shown on the left or the length measured with a stick calibrated in millimeters as shown on the right? Why? The length measured with the millimeters is more precise because it has smaller divisions therefore the measurement will have more decimal places. _________________________________________________________________ 20. How many significant digits are in each of the following measurements? a. 3809 m __4______________________ b. 9.013 m __4______________________ c. 0.0045 m __2______________________ 21. Express the measurements in scientific notation. a. 142000 s __1.42 x 105 s______________________ b. 0.00809 kg __8.09 x 10-3 kg____________________ c. 501000000 m __5.01 x 108 m______________________ Simplify the following expressions. Give your answers in scientific notation, using the correct number of significant digits. 22. (2x106m)(5x105m) 23. (5.06x102m)(8.124m)(1.2x106m) 1 x 1012 m2 4.9 x 109 m3 24. Which of the following measurements contains zeros that are not significant? Give a reason for your answer. 3.050x105mm , 0.0053 m, 45.020 cm, 101.2 g The measurement 0.0053 m has zeroes that are not significant because they are leading zeroes._________________________________________________ _________________________________________________________________ 25. The total mass of four containers is 5.000 kg. If the mass of container A is 256 mg, container B is 5117 cg, and container C is 382 g, what is the mass of container D? (hint: use conversions to make all units the same!) D = 4.57 kg or 4.57 x 103 (4570) g Complete the missing parts of the chart: Symbol Prefix Power of 10 G giga- 109 M mega- 106 k kilo- 103 h hepto- 102 da deca- 101 d deci- 10-1 c centi- 10-2 m milli- 10-3 µ micro- 10-6 n nano- 10-9