Chem 310 - Chemistry Courses
advertisement
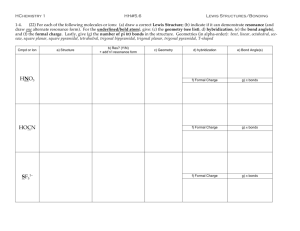
Chemistry 310 - Inorganic Chemistry - Spring 2015 Instructor: Tom Mallouk Cell phone: 814-571-6115 Office hours TR 1:30-2:30 PM 205 S. Frear Lab Office phone: 814-863-9637 Course website: http://courses.chem.psu.edu/chem310 Text: Chem 310 Wikibook (Introduction to Inorganic Chemistry) Other textbooks (recommended, not required): Shriver & Atkins Inorganic Chemistry, 5th Ed (used in 412) Miessler & Tarr, Inorganic Chemistry Huheey, Inorganic Chemistry Chem 310 is part of a two-semester sequence in inorganic chemistry: Chem 310: (Fall and Spring semesters) Bonding models, coordination chemistry Acid-base and redox chemistry Solid state and materials chemistry Nanomaterials and applications Chem 412: (Fall semesters only) Symmetry and group theory Inorganic spectroscopy Organometallic chemistry Catalysis and reaction mechanisms Bio-inorganic chemistry Important concepts: Octet rule Formal charge Resonance Shapes of molecules (VSEPR) Isoelectronic principle Bond polarity and bond strength Lewis dot structures: based on stable s2p6 (octet) configuration : H : :N : H H = H :N H H Formal charge To get formal charge, divide bonds equally between atoms. E.g., NH3 N has 5 e} both atoms are neutral H has 1 e- H + - F H N B F H F H :O H H + - - O O S O- 2+ O- Formal charge is NOT the same as the oxidation state or the actual charge on the atom. Formal charges must add up to overall charge on molecule. Formal charge Low formal charges are most stable. Can be used to tell if a proposed structure is correct or likely. F E.g., BF3 F B F F + F Non-octet + F N O - vs. B - F Octet form is unlikely, because + charge is on F, which is more electronegative than B. Octet O N F More reasonable Resonance Structures + Ozone - O O + O O O O - Terminal O atoms are spectroscopically equivalent Real (instantaneous) structure is average of two forms: + -1/2 O O O -1/2 Resonance Structures - another example O Nitrate ion O N - O + O - + 2 other forms all O’s equivalent O N + O -2/3 bond order 1.33 Resonance Structures: Non-equivalent structures OCN(cyanate ion) -O C N O C Which of these is not an important resonance structure? N- +O C N2- • High formal charge • O is more electronegative than N Real electronic distribution is a weighted average of the first two non-equivalent resonance structures Resonance Structures: No-Bond Resonance +X X Y Z Y Z- ONF3 Electron diffraction shows unusually long N-F bond. Why? F -O N F + F O N F- + 2 other equivalent forms + F F BH3 + CO Lewis acid Lewis base H H B H C O + H+ H B C O H (long B-H bond) Resonance Structures: Hypervalent Compounds e.g., I - + I 2 → I3- hypervalent structure: triiodide ion .. ....- .. :I.. - ..I - ..I: central I has 10 e- (violates octet rule) Vibrational spectra show I-I bond is weaker than in I2 I I I- I- I .. .. octet I = -1/2I ..I I-1/2 bond order = 1/2 Resonance Structures: Hypervalent Compounds XeF2: Same number of valence electrons as I3-1/2F + Xe F-1/2 XPS shows partial (-) charge on F IR shows long (weak) Xe-F bond relative to XeF+ + Xe -F single bond (like I2) Resonance Structures: Hypervalent Compounds F SF6: F F S F F F 12 e- around S F F F- S2+ F FF F F + other resonance structures F S2+ F F-1/3 F bond order = 2/3 Resonance Structures: Hypervalent Compounds Valence shell d-orbitals can be used to create “expanded” octets for 3rd and higher periods: F F F S F F F O- O- O S O O Cl O O- O- + 3d orbitals are high in energy They participate somewhat in bonding, but most molecular properties (shape, bond orders…) are adequately explained using only s and p hybrids. The Isoelectronic Principle Molecules and ions with the same number of valence electrons have similar geometries and properties e.g., O H2C C O CO2 C allene CH2 - - N N + N O N2O + N N - azide ion All four have 3 heavy atoms, 16 valence electrons The Isoelectronic Principle Which one of these does not belong?? F F F B N F F F F O C O F O N - O + O - O C Which of the following is (are) isoelectronic with H2O?? CH4 NH3 F- OH- BH4- - O - The Isoelectronic Principle works for solids too Al Si P S Cd Ga Ge As Se In Sn Sb Te CdSe, GaAs, Ge are all isoelectronic semiconductors InP, AlSb are similar too AlPO4 - same structures as SiO2 similar physical properties Electron pair repulsions can be used to rationalize molecular shapes (Valence Shell Electron Pair Repulsion Theory) Rules: Lone pairs + atoms define “total coord. no” of a central atom. Lone pairs + bonds orient in space to minimize repulsion Repulsive interactions are lp-lp > lp-b > b-b Total coord # 2 3 Shape 180o linear triangular e.g., CO 2 120o e.g., NO 3- Total coord # Shape 4 tetrahedral 5 trigonal bipyramidal (more common) 109.5o CH4 90o equatorial 120o PF5 axial square pyramidal (less common) 6 octahedral Sb(Ph)5 90o SiF62- Examples: O C O 2 bonds to central atom, no lone pairs Total coord. # = 2 (linear) 2 bonding domains + 1 lone pair : SO2 O S + O - Electronic shape is trigonal (CN = 3) Molecular shape is bent O-S-O angle is slightly less than 120o (lone pair repulsion) H H2O 104.5o H Electronic shape: tetrahedral Molecular shape: bent - Examples: - O O S O- 2+ Tetrahedral, no lone pairs on S O- : --- Total C.N. = 5 F F-1/2 Bond order = 1/2 Xe+ - F : --- Xe+ : F -1/2 F- : F - Xe+ F- XeF2 : Xe : F Axial bond order = s1/5pz1/2 = 0.7 (formal charge = -0.3) Equatorial bond order = s1/5px,y2/3 = 0.867 (f.c. = -0.133) (equatorial bonds are less ionic) More electronegative ligands (F) go to axial sites in TBP, so molecule is linear. F- + other resonance forms F F : : F- Br+ : F Br+ : BrF4- F F F Formal charge = -1/2 : : Bond order = 1/2 or ? : Is geometry Molecule is square planar Minimizes lp-lp repulsion 1.68 Å : BrF5 1.78 Å Show that no-bond resonance predicts shorter axial than equatorial bonds Pauling introduced the concept of electronegativity (χ) to explain the extra bond energy of polar molecules H-H H-H O=O H H A-A + B-B Homonuclear diatomics or single bond in more complex molecule O O H H ∂+ ∂2 A-B Very exothermic, but number of bonds is the same (4) on each side. Extra bond energy from electrostatic attraction Bond energies for single bonds are E(AA), E(BB), and E(AB) E(AB) = 1/2[E(AA) + E(BB)] + 23 (χ A - χ B)2 (kcal/mol) Pauling scale for χ is 0.7 (Fr) to 4.0 (F) If Δχ > 2.0, the bond is ionic If 0.5 < Δχ < 2.0, the bond is polar covalent And if Δχ < 0.5, the bond is non-polar. Linus Pauling The polarity of bonds explains something about reactivity: ∂- ∂+ ∂+ ∂e.g. Si - H bond is more hydride-like than C - H 1.8 2.1 2.5 2.1 So silanes react with strong acids to make H2, but phosphines and C-H compounds do not. Electrophilic substiution reactions occur easily on Si - H, P - H cmpds 2.1 2.1 For many bonds, Pauling’s formula is obeyed: D(n) = D(1) - 0.6 log(n) D(1) = single bond length (in Å), D(n) = length of bond order n e.g., C-C bond in alkanes is 1.54 Å C=C (ethane) 1.36 1.33 C≡C (acetylene) 1.25 Å predicted 1.20 observed Some bond energies and bond lengths are anomalous e.g., F-F bond in F2 F covalent radius is 0.64 Å, but F-F bond length is 1.43 Å (extra 0.15Å) calc’d bond order 0.6 X F Cl Br I E(XX) (kcal/mol) 38 Low bond dissociation energy makes F2 58 a very powerful oxidizing agent 46 (oxidizes Xe, Kr, O2) 36 .. .. :F - F: .. .. 1s2 core Lone pair repulsion stretches F-F bond Cl2 is “normal” because core is larger covalent radius = 0.99 Å bond distance = 1.98 Å Discussion topics for Wednesday’s class: •Inequivalent resonance structures •No-bond resonance and hypervalency •Valence bond theory for odd-electron molecules