ENGR 1310 Lecture 18 - Temperature and Heat
advertisement

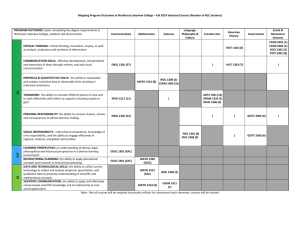
Temperature, Heat, & Combustion EGR 1301: Introduction to Engineering EGR 1301 Models • “A system of postulates, data, and inferences presented as a mathematical description of an entity or state of affairs” • Quantitative approximation of reality Source: Merriam-Webster .com, 2010 Mathematical equations Computer simulations Physical scale models • Why do we use them? Reality is too complex!!! EGR 1301 Energy Conversion Tables “For those who want some proof that physicists are human, the proof is in the idiocy of all the different units which they use for measuring energy.” Richard Feynman Source: Foundations of Engineering, Holtzapple & Reece, 2003 EGR 1301 Utility of Energy for Analysis Source: http://www.fullspectrumsolutions.com/26w_powercompact_65_prd1.htm • Incandescent bulb Resistance heating in filament Light • When filament reaches sufficiently high temperature Light is radiated • Fluorescent bulb Stream of electrons collide with Hg electronsLight • Generates very little heat 60 Watts of electricity 23 Watts of electricity • 800 lumens of light • ~10 cals of heat • 800 lumens of light • ~1 cal of heat EGR 1301 Temperature – What Is It? • NOT the same as HEAT • A quantitative measure of “hotness” • More accurately described on an atomic scale Measures vibrational kinetic energy EGR 1301 Temperature vs. Heat • Temperature A measure of the intensity of internal energy in a system (gas, liquid, or solid) • Heat A measure of the total quantity of thermal energy flow into or out of a system EGR 1301 Temperature vs. Heat • Example: A cup of water at 60°C has much less energy than a hot water heater full of water at 60°C. BUT, the intensity of heat is the same. EGR 1301 Heat Capacity • Energy required to raise temperature of matter by one degree (at constant pressure or constant volume) Q C mT Q = energy in calories m = mass in grams ΔT = temperature change in degrees (C or K) EGR 1301 Constant Pressure Heat Capacities Source: Foundations of Engineering, Holtzapple & Reece, 2003 EGR 1301 Converting Work into Heat: Joule’s Experiment W Fx F ma Q CmT U Q Source: Foundations of Engineering, Holtzapple & Reece, 2003 W Q Ek E p U Win Wout Qin Qout M in M out EGR 1301 Heat Capacity Example Problem • In Joule’s experiment, Beaker contains 5 kg of water Mass spinning the stirrer is 90 kg (g=9.81m/s2) The water increases in temperature by 0.1°C How far did the mass travel? Q CmT F ma W Fx W Q EGR 1301 Heat Capacity Q CmT W Q F ma W Fx 1000 g 1.00cal 5kg Q 0.1C 500cal g C 1kg kg m 1 W Q 500cal 4.184 J s x F ma 90kg 9.81m 1cal 1J s 2 2 x 2.4m EGR 1301 2 States of Matter Source: Foundations of Engineering, Holtzapple & Reece, 2003 EGR 1301 Phase Diagram Source: Foundations of Engineering, Holtzapple & Reece, 2003 EGR 1301 Phase Change • Constant temperature process of transition between phases Melting / Solidification Boiling (vaporization) / Condensation EGR 1301 Phase (or State) Change Energy Qvap mH vap Q fus mH fus • Where m = mass (kg) ΔHvap = latent heat of vaporization (kJ/kg) ΔHfus = latent heat of fusion (kJ/kg) EGR 1301 Phase-Change Energy Source: Foundations of Engineering, Holtzapple & Reece, 2003 EGR 1301 Combustion • Similar to phase change Qcomb mH comb • Where Qcomb = energy released (MJ) m = mass (kg) ΔHcomb = specific heat of combustion (MJ/kg) • Table 22.4 EGR 1301 Example 1: Phase-Change Energy • When water changes from solid to liquid, it must absorb 333.56 kJ/kg from the surroundings What is the energy absorbed to melt ice in units of cal/g? kJ 1kg 1000 J 0.2390cal 333.56 kg 1000 g kJ 1J cal 79.7 g EGR 1301 Example 2a: 1st Law of Thermodynamics • If you have 100 g of water at 22°C and add 20 g of ice at 0°C, what will be the temperature of the 100 g of water once all the ice has melted to form 20 g of water at 0°C? Given : m 100 g m 20 g T 22C T 0C 1 1 2 2 EGR 1301 Example 2a: 1st Law of Thermodynamics Given : m 100 g m2 20 g T 22C T2 0C 1 1 Eqns : Q CmT H fusion mH 1.00cal 79.7cal 100 g T 20 g g C g T 15.94C T 22 15.94C 6.06C 1b EGR 1301 Example 2b: 1st Law of Thermodynamics • What will be the final temperature when the system temperature is uniform (i.e., water from melted ice has warmed and surrounding water has further cooled so that all water is at one temperature)? Given : m1 100 g T1b 6.06C m2 20 g T2 0C 1.00cal 100 g (T 6.06C ) g C 1.00cal 20 g (T 0C ) g C f f T 5.05C f EGR 1301 Example 3: Latent Heat • If the latent heat of vaporization for water is 2,256.7 kJ/kg, what is the latent heat for water in cal/g? cal kJ 0.2390cal 2256.7 539.4 kg 1J g EGR 1301 Example 4a: 1st Law of Thermodynamics • If by sweat and evaporation, you lose 0.031 slugs of water during exercising, how many calories of energy in the form of heat is removed from your body? Weight (mg) of 1 lb is associated with mass of 0.031 slugs Note on p.688 the conversion from slugs to grams 14594 g 539.4cal 0.031slug g slug 244032cal EGR 1301 Example 4b: 1st Law of Thermodynamics • If your body mass is 68,100 g (i.e., 150 lbs), how much would your body temperature rise if you did not sweat and evaporate the sweat in order to cool yourself? Assume the heat capacity for your body is that for water, because your body is ~ 75% water. Q CmT 1cal 244032cal (68100 g )T g C 9 F T 3.58C 6.45 F 5C EGR 1301