presentation ( format)
advertisement

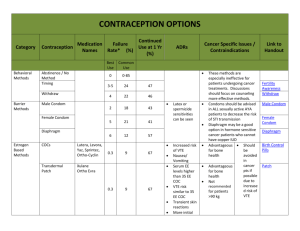
Long Acting Reversible Contraceptive Options for College Women A new era of contraception Beth Kutler FNP Gannett Health Services Cornell University BK82@Cornell.edu The purpose of this activity is to enable the learner to counsel effectively about long acting contraceptive methods (Intrauterine devices and implantable rods) as well as learn new insights for effective placement and management of potential side effects The presenter has no financial conflicts to disclose 2013 ACHA National College Health Assessment : o 49.2% of females had vaginal sex in the 30 days prior to the survey o 1.9 % of college students who had vaginal intercourse within the last 12 months reported experiencing an unintentional pregnancy in that time frame o 18.8 % reported using EC within the last 12 months 7.9% 61.6% 61.7% 2013 ACHA survey 31% Percentage of Women Experiencing Unintended Pregnancy in First Year * Standard Days Method: 5%, Two Day Method: 4% Hatcher RA. Contraceptive Tech. 19th ed. 2007. Increased use of LARC* has the potential to lower unintended pregnancy rates among adolescents *LARC = Long-Acting Reversible Contraception Get It And Forget It ! Contraceptive Cohort Study • Recruited 10,000 participants over 4 years – 60 % age 14-25 – 47% nulliparous – No cost contraception for 3 yrs – Counseled in all methods, starting with top tier methods – Participant choice www.choiceproject.wustl.edu LARC Acceptance Percentage LNG-IUS CuT380A Implant DMPA Pills Ring Patch Other 46.0% 11.9% 16.9% 6.9% 9.4% 7.0% 1.8% <1.0% 75% 10 Method Choice ages 14-20 60 50 40 30 United States 20 Choice 10 0 % of all contraceptive users 2010 % of 4,167 Choice sample www.choiceproject.wustl.edu Intrauterine Contraception in Nulliparous Women a Prospective Observational Study Study Objectives: Assess nulliparous women’s satisfaction with the IUD as a method of contraception Identify any medical history that may predict satisfaction or dissatisfaction with this method Quantify the rates of discontinuation and/or complications for IUD use among nulliparous women 117 women currently enrolled in ongoing study. Receive surveys at 1 ,6, 12 and 18 months following placement What, if any, symptoms did you feel related to the IUD placement ? # Question None Mild Moderate Severe Response Average Value 1 Nausea from the pre-medication 80 6 3 3 92 1.23 2 Cramping from the pre-medication 69 14 6 2 91 1.35 3 Pain and cramping during the IUD insertion procedure 1 23 32 37 93 3.13 4 Light-headedness, nausea, and/or sweating during procedure 49 18 22 4 93 1.80 5 Pain and cramping in the first hour after insertion 2 25 35 31 93 3.02 6 Pain and cramping in the first 24 hours after insertion 4 33 37 19 93 2.76 7 Pain and cramping 24-72 hours after insertion 23 40 18 12 93 2.20 8 Pain and cramping after one week had passed 57 18 14 4 93 1.62 How well informed did you feel prior to your IUD placement ? # Answer Bar Response % 1 Very well informed 80 86.02% 2 Fairly well informed 12 12.90% 3 Neutral 0 0.00% 4 Not well informed 1 1.08% 5 Very poorly informed 0 0.00% 93 100.00% Total How likely are you, at this point (6mo) are you to recommend the IUD to a friend ? # Answer Bar Response % 1 Very Likely 68 79.07% 2 Likely 11 12.79% 3 Neutral 6 6.98% 4 Unlikely 0 0.00% 5 Very Unlikely 1 1.16% 86 100.00% Total But what about…? • • • • Multiple sexual partners Nulliparity Future fertility Placement trouble IUDs Do Not Cause Infertility • Infertility is not more likely after IUD discontinuation compared to other reversible methods • No evidence that IUD use is associated with subsequent infertility • Chlamydia, not previous IUD use, is associated with infertility Safety: IUD Does Not Cause Infertility • IUD is not related to infertility • Chlamydia is related to infertility Odds Ratio 10 1 0.1 Hubacher D, et al. NEJM. 2001. Tubal infertility by previous copper T IUD use and presence of chlamydia antibodies, nulligravid women Fertility Rates in Parous Women After Discontinuation of Contraceptive 100 Pregnancies (%) 80 IUC 6060 OC Diaphragm 40 Other methods 20 0 0 12 18 24 30 Months After Discontinuation Vessey MP, et al. Br Med J. 1983. Andersson K, et al. Contraception. 1992. Belhadj H, et al. Contraception. 1986. 36 42 Ectopic Pregnancy • IUDs may be offered to women with a history of ectopic pregnancy (MEC cat. 1) • IUD use does not appear to increase absolute risk o Ectopic rate with IUD= 0.5/1,000 women-years o Ectopic rate with no contraception= 3.25-5.25/1,000 women- years o However, if pregnancy does occur with an IUD in place, the pregnancy is more likely to be ectopic Sivin I. Dose- and age-dependent ectopic pregnancy risks with intrauterine contraception. Obstet Gynecol 1991;78:291–8. Ectopic Pregnancy Levonorgestrel IUS 0.20* Copper IUD 0.34* No method 1.20-1.60* *Ectopic pregnancies per 1,000 woman-years Andersson et al. Contraception 1994;49:56. Sivin. Stud Fam Plann 1983;14:57-63. Safety: IUDs Do Not Cause PID • PID incidence for IUD users is similar to that of the general population • Risk is increased only during the first month after insertion • Preexisting STI at time of insertion, not the IUD itself, increases risk Svensson L, et al. JAMA. 1984. Sivin I, et al. Contraception. 1991. Farley T, et al. Lancet. 1992. Rate of PID by Duration of IUD Use Rate per 1,000 woman years N = 20,000 women 9.25 1.6 <21 days of use Adapted from Farley T, et al. Lancet. 1992. 21 days - 8 years of use Screening: Poor Candidates for Intrauterine Contraception • • • • • • Known or suspected pregnancy Puerperal sepsis Immediate post septic abortion Unexplained vaginal bleeding Cervical or endometrial cancer Uterine fibroids that interfere with placement • Current purulent cervicitis, chlamydia, or gonorrhea WHO. Medical Eligibility Criteria for Contraceptive Use. Copper IUD • ParaGard polyethylene wrapped with copper wire • Approved for use up to 10 years (probably more) • Mechanisms of action: Inhibition of sperm migration and viability Change in ovum transport speed Damage to or destruction of ovum Damage to or destruction of fertilized ovum All effects occur before implantation • Highly effective LNG IUS • Mirena LNG IUS releases 20 mcg levonorgestrel/day • Approved for use up to 5 years (probably more) • Mechanisms of action: Similar effects as copper IUD Also causes endometrial suppression and changes in cervical mucus All effects occur before implantation • Highly effective New LNG IUS 3.3 cm sq 2.8 x 3 cm Insertion tube o.5 cm Insertion tube 0.4cm • Skyla LNG IUS releases 14 mcg levonorgestrel/day • Approved for use up to 3 years • Highly effective IUD Placement Screening for STI chlamydia and gonorrhea 57,728 IUD insertions: • 47% unscreened • 19% screened on day IUD was inserted • Overall PID risk within 90 days= 0.54% • No difference in PID regardless of screening Suffrin et al OB. Gyn 2012 120: 1314-21 Screen at risk women through most convenient process • Pre placement counseling essential • 800 mg Ibuprofen 1 hour prior to placement ± • • • • • • Anxiolytics 50 mg Tramadol Topical and/or instilled lidocaine Cervical blocks Misoprostol Menses Post-Abortion Insertion • Insertion of an IUD immediately after abortion or miscarriage is safe and effective – Significantly reduces the risk of repeat abortion – Increases rates of use – Adolescents should be counseled regarding risk of expulsion Copper IUD as EC • Most effective method of emergency contraception • Can be inserted up to 5 days after unprotected intercourse to prevent pregnancy Efficacy of Emergency contraception Glasier A, et. all. Contraception. 2011;84:363‐7 . Bleeding Concerns Fertility and Sterility Volume 97, Issue 3, March 2012, Pages 616–622.e3 Copper T related bleeding can decrease over time Side effects from the copper IUD: do they decrease over time? David Hubacher⁎, Pai-Lien Chen, Sola Park. Contraception 79 (2009) 356–362 Managing Bleeding Concerns • Anticipatory guidance and reassurance • Treat with NSAIDs • Cycle with oral contraceptives • 70% experience oligomenorrhea or amenorrhea within 2 years of insertion of Mirena • These numbers likely to be lower with Skyla 3 most common reasons for requesting removal before 6 months of use Obstet Gynecol 2013;122:1214–21) Nexplanon Convenient Long acting reversible method. Discrete. Effective for 3 years Effective 0.5 to 1 pregnancy /1,000 users Reversible After implant is removed, most women (94%) ovulate by 3 months; the majority ovulate within 3 weeks. Drug level is undetectable one week after removal. Funk S, Contraception. 2005 May;71(5):319-26. Safe Progesterone only. MEC 1 or 2 where estrogen is contraindicated. Inhibits ovulation No evidence of long-term effects such as deep vein thrombosis or anemia. Studies regarding bone mineral density have been conflicting. BeerthuizenR, Hum Reprod. 2000;15:118-122. Easy Placement • • • • Insertion in <1 min Removal 3-5 min 1% complications related to insertion 1.7% related to removal • • Can be placed anytime pregnancy reasonably excluded Back up method for 7 days unless : • inserted within 5 days of menses • immediately post-abortion • Immediately upon switching from another hormonal method Bleeding patterns with etonogestrel implant Contraception Volume 71, Issue 5, May 2005, Pages 319–326 Bleeding Patterns with Implant First 2 Years Frequent Prolonged Amenorrhea Infrequent Percentage of 90– day intervals 6.1% 16.9% 21.4% 33.3% Managing Bleeding Concerns • Common strategies include short courses of combined OCs or NSAIDs – No published placebo controlled trials to support use of these treatments • Limited data suggest decreases in bleeding episode length with: – Mefenamic acid – Mifepristone in combination with ethinyl estradiol or doxycycline – Doxycycline alone Weight Changes with LARC Methods Contraception 88 (2013) 503–508 Thank You ! Questions ? Beth Kutler BK82@cornell.edu LARC Online Resources www.acog.org/goto/larc www.jahonline.org www.arhp.org http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/USSPR.htm Selected practice recommendations for contraceptive use, (SPRC, 2013) http://www.cdc.gov/reproductivehealth/UnintendedPregnancy/USMEC.htm Medical Eligibility Guidelines for Contraception (MEC, 2010) www.bedsider.org