Chem131-Ch1
advertisement

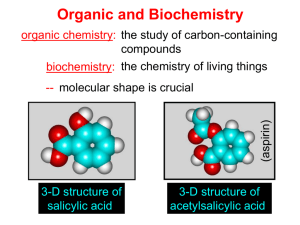
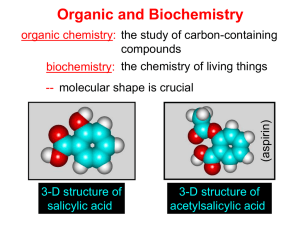
Chapter I Hydrocarbone Nomenclature and Reaction Organic compounds are compounds that could be obtained from living organisms. Inorganic compounds are compounds that came from nonliving sources. Introduction Organic Chemistry The chemistry of the compounds of carbon The human body is largely composed of organic compounds Organic chemistry plays a central role in medicine, bioengineering etc. Vitalism It was originally thought organic compounds could be made only by living things by intervention of a “vital force” Fredrich Wöhler disproved vitalism in 1828 by making the organic compound urea from the inorganic salt ammonium cyanate by evaporation: Chapter 1 3 There are more than 10 million compounds listed in Chemical Abstracts, and most of these are organic. Fortunately we can classify them into a few dozen families. This classification is based on structural entities which have a chemical reactivity that is roughly predictable - socalled functional groups. functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon Alkanes There are various subdivisions in the classification of hydrocarbons. One family of hydrocarbons is called the alkanes, or sometimes paraffins (from the Latin parum affinis, meaning "little affinity" and thus implying a lack of reactivity) or aliphatics (from the Greek word aleiphar meaning "fat or oil"). They are also called saturated hydrocarbons. All alkanes that are open chain (not rings) have the general formula CnH2n+2, where n is an integer. Alkanes : CnH2n+2 Name Methane Ethane Propane Butane Pentane Hexane Heptane Octane Nonane Decane Molecular Formula CH4 C2H6 C3H8 C4H10 C5H12 C6H14 C7H16 C8H18 C9H20 C10H22 Classes of carbon and Hydrogen • • • • • Primary carbon : CH3-R Secondary carbon : R-CH2-R Tertiary carbon : (R)2-CH-R Quaternary carbon: (R)4-C Hydrogens are also referred to as 1º, 2º or 3º according to the type of carbon they are bonded to. Methane Alkyl groups • Alkyl groups are named by dropping the -ane suffix of the alkanes and adding the suffix -yl. Methane becomes a methyl group, ethane an ethyl group, etc. Alkanes Alkyl groups + aneاألسم األساسي (المقطع االغريقي) methane meth + ane IUPAC system of nomenclature 1. Find and name the longest continuous carbon chain. 2. Identify and name groups attached to this chain. 3. Number the chain consecutively, starting at the end nearest a substituent group. 4. Designate the location of each substituent group by an appropriate number and name. 5. Assemble the name, listing groups in alphabetical order. 6. The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing. 7. Halogen substituent's are easily accommodated, using the names: fluoro (F), chloro (Cl-), bromo (Br-) and iodo (I-) also (-NH2) amino, (-NO2) nitro… 1- Locate the longest continuous chain of carbon atoms; this chain determines the parent name for the alkane. Sometimes, you may need to go around corners and zigzag to find the longest (parent) chain. (the parent chain is in blue): • CH3 If the parent chain for example has 6 carbon atoms, therefore, it is a derivative of hexane and if it has 4 carbon atoms it is derivative of butane and so on . H H3C CH CH2 C CH3CH2CH2CH2CHCH3 CH3CH2CH2CH2CHCH3 CH3 CH2 CH3 18 H2C CH2 CH3 CH3 2- Number the longest chain beginning with the end of the chain nearer to the substituent. Substituent 6 5 4 3 2 1 CH3CH2CH2CH2CHCH3 Substituent CH3 7 6 5 4 3 CH3CH2CH2CH2CHCH3 2 CH2 1CH3 19 3- Use the numbers obtained by application of rule 2 to designate the location of the substituent group. The parent name is placed last; the substituent group, preceded by the number indicating its location on the chain, is placed first. 20 4. When two or more substituents are present, give each corresponding to its location substituent a number on the longest chain. The substituent groups are listed alphabetically regardless of their order of occurrence in the molecule. 21 5) When two or more substituents are identical, indicate this by the use of the prefixes di-, tri-, tetra-, and so on. 22 6) When two substituents are present on the same carbon, use the number twice. CH3 H3CCH2 C CH2CH2CH3 CH2 CH3 3-Ethyl-3-methylhexane 23 7. When two chains of equal length compete for selection as the parent chain, choose the chain with the greater number of substituents. 24 8. When branching occurs at an equal distance from both ends of the longest chain, choose the name that gives the lower number at the first point of difference. 25 Drawing alkanes CH3-CH2-CH2-CH2-CH3 n-Pentane Physical Properties • Methane, ethane, propane, and butane are gases; pentane through hexadecane are liquids; the homologues larger than hexadecane are solids. • The boiling points of alkanes increase with molecular weight. • Branching reduces the boiling point, the more branching the lower the boiling point. • Alkanes are almost completely insoluble in water. Reaction of alkanes 1-Halogenatio 2- combustion of alkanes Cycloalkanes : •Cycloalkanes are alkanes that have carbon atoms that form a ring (called alicyclic compounds) •Simple cycloalkanes are rings of (CH2)n, or CnH2n Naming Cycloalkane Count the number of carbon atoms in the ring and the number in the largest substituent chain. If the number of carbon atoms in the ring is equal to or greater than the number in the substituent, the compound is named as an alkyl-substituted cycloalkane For an alkyl- or halo-substituted cycloalkane, start at a point of attachment as C1 and number the substituents on the ring so that the second substituent has as low a number as possible. Number the substituents and write the name 32 Alkenes Alkenes area class of HYDROCARBONS which contain only carbon and hydrogen. Two other terms which describe alkenes are unsaturated and olefins. UNSATURATED hydrocarbons contain either double or triple bonds. Since the compound is unsaturated with respect to hydrogen atoms, the extra electrons are shared between 2 carbon atoms forming double bonds in alkenes. Alkenes are also called OLEFINS because they form oily liquids on reaction with chlorine gas. All alkanes that are open chain (not rings) have the general formula CnH2n, where n is an integer HYBRIDISATION OF ORBITALS - ALKENES + 3 SP2 P Trigonal Planar π σ Nomenclature of alkenes 1. The ene suffix indicates an alkenes or cycloalkenes. 2. The longest chain chosen for the root name must include both carbon atoms of the double bond. 3. The root chain must be numbered from the end nearest a double bond carbon atom. If the double bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts. 4. In cycloalkenes the double bond carbons are assigned ring locations C1 and C2. Which of the two is C1 may be determined by the nearest substituent rule. 5. Substituent groups containing double bonds are: 38 H2C=CH– Vinyl group H2C=CH–CH2– Allyl group 3-Propyl-2-heptene 2,3-DiMethyl cyclohexene 3-Chloro-4-ethyl cyclobutene 3-Methyl cyclohexene Physical Properties of Alkenes Alkenes are non polar compounds. Insoluble in water. Soluble in non polar organic solvents. They are less dense than water. Range of physical states: ≤ 4 C's are gases 5 - 17 C's are liquids ≥ 18 C's are solids The alkenes has a boiling point which is a small number of degrees lower than the corresponding alkanes. 41 Reaction of alkene 1-Butene n-Butane 1-Butene 1,2-DiChlorobutane 2-Butene 2-Butene 2-Clorobutane 2-Hydroxybutane 1-Butene 1-Butene 2-Chlorobutane 1-Chlorobutane 2-Hydroxybutane shape: Linear Nomenclature 1. Identify the longest continuous chain of carbon atoms that contains the carbon-carbon triple bond. the - ane ending is changed to – yne 2. Number the carbon atoms of the longest continuous chain, starting at the end closest to the triple bond. . 3. The location and name of any substituent atom or group is indicated. 51 Reaction of alkyne Alkenes and alkynes are generally more reactive than alkanes due to the electron density available in their pi bonds 1. Addition of halogen 2-Addition of hydrogen 3. Addition of hydrogen halide Reaction of alkyne ketone Acidity of alkyne More acidity Aromatic Compounds Benzene • Benzene (C6H6) is the simplest aromatic hydrocarbon (or arene). • Benzene has four degrees of unsaturation, making it a highly unsaturated hydrocarbon. Benzene contains a planar 6-membered ring. •All C-Cbond lengths are equal. •Kekuléproposed two equilibrated structures • Whereas unsaturated hydrocarbons such as alkenes, alkynes and dienes readily undergo addition reactions, benzene does not. Nomenclature of Benzene Derivatives monosubstituted benzenes Aside from the trivial names, we usually name monosubstituted benzenes with benzene as the parent. The name of the substituent is prefixed to the parent name. For other monosubstituted benzenes, the presence of the substituent results in a new parent name (Common name). disubstituted benzene A disubstituted benzene can be named with prefix numbers or by the ortho, meta, para system, which shows the positional relationships of the two groups to each other on the ring. Reactions of Benzene Aromatic substitution reactions As described earlier benzene does not undergo addition reactions as do alkenes, but substitution reactions of benzene are common. In these, a group or an atom is substituted for a ring H – hence the reaction is referred to as an aromatic substitution reaction. Halogenation: Treatment of benzene with bromine (Br2) in the presence of an iron(III) halide catalyst yields bromobenzene, and Using chlorine gives chlorobenzene Nitration: If benzene is treated with concentrated nitric acid, with concentrated sulfuric acid as the catalyst, nitrobenzene is formed. Sulfonation: Treatment of benzene with fuming sulfuric acid gives benzenesulfonic acid. Alkylation: When treated with a alkyl halides, denoted by R—X (see next topic) and aLewis acid catalyst (AlX3 in this reaction), benzene is converted to an alkylbenzene. This reaction is called a Friedel-Crafts alkylation after Charles Friedel, a French chemist, and James Crafts, an American chemist, who developed this reaction in 1877. The term alkylation means substitution by an alkyl group. Acylation: Friedel and Crafts developed a reaction similar to the alkylation reaction above. This type of reaction is called a Friedel-Crafts acylation because an acyl group (see later) , not an alkyl group, is substituted on the benzene ring. The symbol "R" is used commonly in organic chemistry to denote an alkyl substituent.