template LAB #7
advertisement

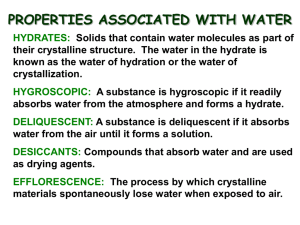
RUBRIC Regents Chemistry Lab # 7 Name: ________________________________Date Due: Tues 11/10/15 Section 1:________/5 Percent Water in a Hydrate Section 2:________/7 Section 3:________/4 The percentage of water in the original hydrate is calculated as follows: % hydrate (water) = mass of water lost x 100 mass of hydrated salt Section 4:________/4 Section 1: Pre-Lab questions (use FCGCS; calculations can be done by hand and SHOW ALL WORK) 1. Explain what is meant by… a hydrated salt? an anhydrous salt? 2. When a hydrate is heated what happens to the water molecules? 3. Calculate the formula mass of BaCl2•3H2O. 4. You have 50.0g of BaCl2 but you need to know how many moles this represents. Show how you would convert the grams to moles. 5. What percent by mass of BaCl2•3H2O is water? Section 2: Data/Calculations a) mass of evaporating dish ________________________ b) mass of evaporating dish + hydrated salt ________________________ c) mass of evaporating dish + anhydrous salt ________________________ d) mass of the hydrate used (b-a) ________________________ e) mass of the anhydrous salt (c-a) ________________________ f) mass of the water lost _________________________ (d-e) g) percent of water in the hydrate ________________________ (answer to the hundreths place) Section 3: Evaluating error (answer Q in FCGCS) 1. The actual value for the percent of water in this hydrate is 36.0%. Determine your percent error using the formula from table T. 2. List THREE possible sources of error in your experiment. (Be Specific!) 3. Why must the evaporating dish be cool when you measure its mass? 4. Why must you determine the mass of the anhydrous salt as soon as it cools instead of waiting 5 or 10 minutes? (hint: this is especially important on a humid day) Section 4: Solving for X (CuSO4•XH2O) (calculations can be done by hand; calc #6 and #7 to THREE decimal places; for #8 round answer for X to whole number)) 5. Determine the molar mass of: a. CuSO4 b. H2O 6. Determine the number of moles of CuSO4 in your sample from your calculated mass of anhydrous salt. 7. Determine the number of moles of H2O in your sample from your calculated mass of water lost. 8. Find the smallest whole number ratio (X) of H2O to moles of CuSO4. Use the proportion below (round answer X to nearest whole number) moles of water (#7 above) moles of CuSO4 (#6 above) = X moles of water 1 moles of CuSO4 FILL IN YOUR ANSWER for X HERE: CuSO4•______H2O