CHEMICAL REACTIONS
advertisement

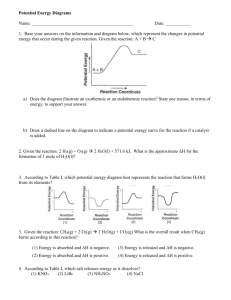
CHEMICAL REACTIONS REVIEW OF CHEMICAL CHANGES Occurs when a substance reacts and forms one or more new substances Examples: bake a cake, leaves change, food digested, food spoils HOW DO YOU KNOW WHEN A CHEMICAL CHANGES HAS OCCURRED? LOOK FOR EVIDENCE! Indicators of chemical reactions Emission of light or heat Formation of a gas Formation of a precipitate Color change Emission of odor CHEMICAL VS. PHYSICAL CHANGE Not always easy to tell! Water boiling to steam is still water Horseshoe heat turns red is still iron Are different substances present after the change takes place? When matter undergoes chemical change, the composition of the matter changes. When it undergoes physical change the composition of the matter remains the same. Chemical reaction is said to take place when a chemical change happens Describe what was present before & after the reaction Reactants: substances that undergo change Products: new substances formed as result of change Example: NaCl + BeF NaF + BeCl2 Reactants Products CHECK YOUR UNDERSTANDING 1. 2. 3. 4. 5. CHEMICAL OR PHYSICAL CHANGE? Boiling water Burning wood Food scraps decomposing Salt dissolving in water Burning propane USING EQUATIONS Reactants Products Carbon + Oxygen Carbon Dioxide C + O2 CO2 Chemical equation: representation of a chemical reaction in which the reactants and products are expressed as formulas. Symbols used in equations (s) after the formula –solid Cu(s) (g) after the formula –gas H2 (g) (l) after the formula -liquid H2O(l) (aq) after the formula - dissolved in water, an aqueous solution. CaCl2 (aq) used after a product indicates a gas (same as (g)) O2 used after a product indicates a solid (same as (s)) CaCo3 CONSERVATION OF MASS Mass is neither created or destroyed in a chemical reaction Mass of products always equals mass of reactants Charcoal burning into pile of ashes – but CO2, too… pg. 193 BALANCING EQUATIONS In order to show that mass is conserved during a reaction, a chemical equation must be balanced! Coefficients: numbers that appear before formula Example: 2 H20 3 C6H12O6 STEPS IN BALANCING 1.Box in the compounds. Then, NEVER change anything in the box. N2H4 + O2 N2 + H2O 2. Set up an element inventory. N H O R 2 4 2 P 2 2 1 3. Add coefficients (the big numbers) in front of the boxes. 4. Always update the inventory with every coefficient. PRACTICE Write a balanced equation for the reaction between copper and oxygen to produce copper (II) oxide, CuO Reactants: Cu, O2 Product: CuO Cu + O2 CuO Cu + O2 2 CuO 2 Cu + O2 2 CuO Look back and ask, “Is your answer reasonable?” TYPES OF REACTIONS Reactions classified by type of reactant or the number of reactants & products SYNTHESIS SINGLE REPLACEMENT DOUBLE REPLACEMENT DECOMPOSITION COMBUSTION RE-DOX (Oxidation – Reduction) SYNTHESIS REACTIONS Reaction in which 2 or more substances react to form a single substance Reactants can be compounds or elements Products are always compounds A + B AB 2Na + Cl2 2NaCl2 2H2 + O2 2H2O DECOMPOSITION REACTIONS Opposite of synthesis Reaction in which a compound breaks down into 2 or more simpler substances Reactant must be compound Products can be elements or compounds AB A + B CaCO3 CaO + CO2 SINGLE REPLACEMENT REACTIONS Reaction in which one element takes the place of another element in a compound A + BC B + AC Cu + 2 AgNO3 Cu(NO3)2 + 2 Ag 2K + 2H2O H2 + 2KOH DOUBLE REPLACEMENT REACTIONS Reaction in which 2 different compounds exchange positive ions and form 2 new compounds AB + CD AD + CB ACTIVITY SERIES Used to predict which elements will replace others Activity Series of Metals: K Ca Mg Zn Fe Pb H Cu Ag Reactivity decreases down the series Fe + CuSO4 FeSO4 + Cu Can predict that iron will replace copper COMBUSTION REACTIONS Reaction in which a substance reacts rapidly with oxygen, often producing heat / light Burning of natural gas (methane) CH4 + 2O2 CO2 + 2 H2O TIP: If the reactants include a fuel & O2and the products include CO2 & H2O, it’s probably a combustion reaction REACTIONS AS e- TRANSFERS Some chemical reactions are transfers of ebetween atoms OXIDATION: any process in which an element loses e-… an element is oxidized if it loses e- Oxygen does not always have to be present REDUCTION: process in which an element gains e- during a chemical reaction… element is reduced if it gains e- Oxidation – Reduction always occur together CHECK YOUR UNDERSTANDING WHAT TYPE OF REACTIONS ARE THESE? Are they balanced? __ Na + ___ Cl2 ___ NaCl __ CH4 + __ O2 __ CO2 + __ H2O __ Na2S + __ AgNO3 _ NaNO3 + _ Ag2S ___ Mg + ___ HCl ___ H2 + ___ MgCl2 ENERGY CHANGES IN REACTIONS CHEMICAL ENERGY: energy stored in the chemical bonds of a substance Propane – C3H8 Has 10 single bonds Chemical reactions involve the breaking of chemical bonds in the reactants and formation of chemical bonds in the products EXOTHERMIC & ENDOTHERMIC REACTIONS During a chemical reaction, energy is either released or absorbed EXOTHERMIC: chemical reaction that releases energy into its surroundings - Combustion ENDOTHERMIC: chemical reaction that absorbs energy from its surroundings - Add the energy term kJ into reactants LAW OF CONSERVATION OF ENERGY Energy is neither created nor destroyed WHAT DOES THIS LAW REMIND YOU OF? REACTION RATES Some reactions happen immediately, others happen over time REACTION RATE: rate at which reactants change into products over time Tells you how fast a reaction is going FACTORS AFFECTING REACTION RATES 1.Temperature: ^ temp will ^ rr - Causes particles to move faster, collide, and react 2. Surface area: increases the exposure of reactants to one another, so more collisions, and more reactions 3. Stirring: ^ exposure of reactants to each other 4. Concentration: number of particles in a given volume 5. Catalysts: substance that affects the rr w/o being used up in the reaction V2O5 - 2 SO2 + O2 --- 2SO3 EQUILLIBRIUM State in which the forward & reverse paths of a change take place at the same rate Physical equilibrium – when physical change does not go to completion, a physical equilibrium is established Ex. Water evaporating in a sealed bottle H2O(l) ↔ H2O(g) Chemical equilibrium- most chemical reactions are reversible to some degree… Reversible reactions are those in which the conversion of reactants into products and conversion of products into reactants can happen simultaneously 2SO2(g) + O2 ↔ 2SO3