Unit 10 Part 1
advertisement

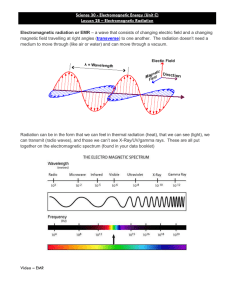
Waves Wave-Particle Duality The electron was previously describe by J.J. Thompson as a particle. He won a Nobel prize for his research His son, George Thompson described the electron as having a wave-like nature He won a Nobel prize for his research Who was correct????? Both! To better understand the current model of the atom we will investigate how the electron acts as a wave but also acts as a particle. Part 1: Waves We will begin our Journey by discussing how an electron propagates through space as an energy wave….. Waves Waves transmit energy through a medium If you throw a stone into the middle of a pond with a smooth surface, it creates a “ripple” on the surface of the water. Ripples = waves The energy from the stone is being transferred through the medium, water, in the form of waves. Waves If a glass bottle is floating on the surface of the water, the waves will move the bottle vertically (up and down) but will not carry the bottle in the direction of the wave. Movement of bottle Direction of wave Properties of Waves Waves can be represented using drawings and mathematical equations. An imaginary line may be drawn horizontally at an equal distance from both the crest and the trough of a wave. The crest of a wave is the top peak of the wave. The wave’s trough is the bottom of the wave. Crest Picture 1 Wave trough Amplitude The amplitude of a wave is the distance from the imaginary line to the crest or trough of a wave. Picture 2 Amplitude Amplitude Frequency The frequency of a wave is the number of waves which pass a given point in a specified unit of time. Picture 3 Frequency Low frequency (ex: 1 wave in 1 sec) High frequency (ex: 3 waves in 1 sec) Frequency The symbol for frequency is the Greek letter nu. Nu = ν The unit for frequency is a hertz, which is abbreviated Hz. One hertz is equal to one cycle per second or sec-1 Wavelength Wavelength is the distance between similar sets of a wave, such as from crest to crest or trough to trough. Picture 4 Wavelength wavelength wavelength Wavelength The symbol for wavelength is the Greek letter lambda. Lambda = λ The most common unit used when expressing wavelength is the meter; however, the unit Angstrom is sometimes used. Angstrom = _____ 1 Angstrom = 1 x 10-8 cm (exactly) Speed of Wave The speed of any wave equals wavelength times frequency. Speed of wave formula: Speed = wavelength * frequency s= λ*ν Wave-Speed calculations 1. A water wave has a frequency of 4.75 x 10-2 Hz and a wavelength of 1.50 x 101 m. Speed = Wave-Speed calculations 2. The speed of a wave is 4.75 m/s and its frequency is 8.35 Hz. Calculate its wavelength. Speed = Rearrange for wavelength Sound vs. Radio Sound Waves A sound wave needs a medium to allow it to spread (air, water, solids etc). Radio Waves Radio Waves can travel through the air or the vacuum of space so they do not need a medium Electromagnetic Radiation Radio waves are considered to be Electromagnetic Radiation (energy), where as sound waves are not. Electromagnetic Radiation is energy that can travel through a vacuum, in the form of waves and at the speed of light. Electromagnetic energy has no mass. Lets take a closer look at Electromagnetic Radiation…. Electromagnetic Radiation Frequencies and wavelength of electromagnetic radiation are related by the speed of light --- all electromagnetic radiation travels at the speed of light, including radio waves. Note: Assume EM waves travel at the speed of light regardless of being in vacuum Speed of light (c) = 3.00 x 108 m/s Speed formula The speed of any wave is equal to the product of its wavelength and frequency (recall: speed = λ*ν) We can use this information for electromagnetic waves as well. Formula can be adjusted slightly…. Speed of light (c) = λ*ν c = λ*ν Formula: c = λ*ν Whenever you are solving problems using the formula given above make certain that all measurements for wavelength are expressed in meters. If the wavelength is given in Angstroms, convert Angstroms to meters than apply the formula 1 Angstrom = 1 x 10-8 cm = 1 x 10-10 m Wavelength is inversely proportional to frequency. If frequency increases wavelength must decrease Electromagnetic Spectrum Electromagnetic waves are produced by a combination of electrical and magnetic fields The electromagnetic waves are organized in an electromagnetic spectrum Each type of The spectrum includes Radio waves Microwaves Infrared Radiation Visible light Ultraviolet rays X-rays Gamma rays electromagnetic radiation is associated with a range of wavelengths and frequencies. Electromagnetic spectrum low energy High energy Visible Light Visible light makes up a small portion of the electromagnetic spectrum Visible light consists of seven different colors ROYGBIV (red, orange, yellow, green, blue, indigo, violet) If red light has the lowest frequency, it must have the greatest wavelength compared to the other colors of the visible spectrum 1nm = 1.0 x 10-9 m 1m = 1,000,000,000 nm Visible Light There are no precise boundaries between the different types of waves that compose the electromagnetic spectrum. However, the following frequencies are associated with the following colors: Wave Frequency in Hz Red light 4.3 x 1014 Yellow light 5.2 x 1014 Blue light 6.4 x 1014 Violet light 7.5 x 1014 Radio Waves Radio stations send out radio waves on a specific frequency. Depending upon the strength of their broadcasting antenna – the listening area may be large or small. No two broadcasting signals may be the same in overlapping areas We go from 88 to 108 FM band. (frequency modulation) These frequencies are in kilohertz which is 103 Hz. The individual frequencies have associated wavelengths – which may be determined and calculated. Wave-Speed calculations 1. A gamma ray has a frequency of 3.75x 1023 Hz. What is the wavelength? 2. What radio station sends out a signal with a wavelength of 3.25m? ROYGBIV The greater the frequency the greater the energy Visible light is made up of ROYGBIV. Each color associates with a different frequency. A light bulb emits all of these frequencies at once and the light appears white. When atoms of an individual element absorb and release energy, scientists assumed the atoms would emit a continuous spectrum, but instead they observed bright lines of colors at specific wavelengths (or frequencies) Why do we see these bright line spectrums instead of a continuous spectrum? Photons Thus far we have seen that Electromagnetic radiation displays characteristics If a beam of light isof waves, but EM radiation also up of these hasmade some properties of particles. small packets/photons Just as water waves transmit energy, why electromagnetic don’t we see waves also transmit energy them? Light energy (EM radiation) comes in tiny packets called photons Energy Levels When an atom absorbs energy, its electrons make transitions from lower energy levels to higher energy levels. The energy absorbed can be in the form of heat (as in a flame) or electrical energy However, when electrons subsequently return from higher energy levels to lower energy levels, energy is released in the form of electromagnetic radiation (light). The same reason why we do not see individual water molecules when you turn on the faucet These packets are traveling at the speed of light. They are moving too fast for our eyes to see the photons and the photons are extremely small. Energy The energy of a photon is proportional to the frequency of the electromagnetic radiation. So, as the frequency of an electromagnetic wave increases, the energy of the photons from that wave will also increase. E increases as ν increases Each frequency has a specific energy. The relationship between energy of a photon and frequency can be expressed by the following mathematical relationship…. Energy of a photon = Plank’s constant * frequency Formula: E=h*ν Symbol for energy is E Unit for energy is Joules abbreviated J A joule is kgm2 s2 The symbol for plank’s constant is h Plank’s constant is equal to 6.6262x10-34 J*s If the frequency of electromagnetic radiation is directly proportional to the energy of a photon, then the energy of the photon must be inversely related to the wavelength. Recall that the frequency is inversely proportional to wavelength. So as wavelength increases, frequency decreases and so does energy. c= λ*ν E=h*ν What is the connection between these two formulas? De Broglie’s Equation E= hc λ The spacing between energy levels in an atom determines the size of the transitions that occur, and thus the energy and wavelengths of the collection of photons emitted. When electrons return from higher energy levels more energy is released than when electrons return from lower energy levels. The colors in a bright line spectrum indicate the energy levels from which electrons are returning . Colors with lower frequency (red) indicate less energy which indicates the return from lower energy levels.