SCH4C Name: Lab 1: Identifying a Mystery Powder Background
advertisement

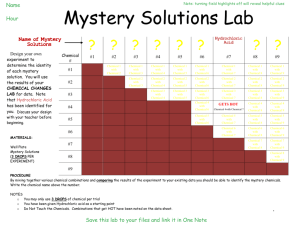
SCH4C Name: ________________________ Lab 1: Identifying a Mystery Powder Background: Forensic science applies science to investigate crime scenes. Forensic scientists collect, analyze and interpret evidence, often using qualitative analysis. Qualitative analysis is the identification of a substance using of physical and chemical properties. In this lab, imagine you are forensic scientists trying to identify a white powder found at a crime scene. You will use qualitative analysis to identify the substance. Question: What is the identity of a mystery powder? Materials: eye goggles lab apron disposable gloves 6 white powders (A to F) mystery powder ____ 3 microtrays eyedroppers scoopula toothpicks deionized (DI) water universal pH indicator dilute hydrochloric acid, HCl (aq) dilute iron (III) nitrate solution, Fe(NO3)3 (aq) iodine solution, I2 (aq) Procedure: 1. Read the procedure and create an observation table in your lab book. 2. Place two microtrays side by side on the bench. Label the columns A to E and the rows 1 – 4. 3. Using a scoopula, place a small amount of the substance A to the four well in column A. Place samples A, B, C, D, E and F in the other columns. Observe and record the appearance of each powder. 4. Using an eyedropper, add 5 drops of DI water to the six samples (A to F) in row 1. 5. Using an eyedropper, add 1 drop of universal indicator to the sample/water in the first row of the microtray. Mix each sample with a clean toothpick. Record your observations. 6. Using an eyedropper, add 5 drops of dilute hydrochloric acid to each sample In the second row. Mix the contents of each well with a toothpick. Record your observations. 7. Using an eyedropper, add 5 drops of dilute iron (III) nitrate to each sample in the third row. Mix the contents of each well with a toothpick. Record your observations. 8. Using an eyedropper, add 5 drops of iodine solution to each sample in the fourth row. Mix the contents of each well with a toothpick. Record your observations. 9. Obtain another microtray and a mystery powder. Repeat steps 2 – 7 with the mystery powder. 10. Place the waste in the appropriate container and clean up. Analysis and Conclusion: 1. Write a statement that answers the question in this lab. 2. What are two additional tests that could be carried out to improve your level of confidence in your answer to A. 3. Describe two possible sources of error that could lead to identifying the wrong substance. Title of the Lab Date Purpose: Rewrite the purpose. Materials and procedure: Refer to handout. Observations: Create a blank table. Sample A B C D E F Mystery Appearance Universal Indicator Colour Reaction with HCl Reaction with Fe(NO3)3 Reaction with I2