Tuesday 3/26/13: PREDICTING PRODUCTS
advertisement

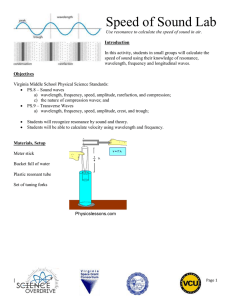
Tuesday 3/26/13: PREDICTING PRODUCTS REVIEW INFORMATION: A. Equations show: 1.) The reactants that enter a reaction 2.) The products that are formed by the reaction (rxn) Example: CO2 + H2O H2CO3 Reactants Product B. Predicting Products 1.) The products of a chemical reaction may often be predicted by applying known facts about common reaction types. 2.) There are hundreds of different ”kinds” of chemical reactions, only four general types of reactions will be considered: single displacement, double displacement, decomposition, and synthesis. Single Displacement General form of a single displacement: element + compound element + compound A + BC AC + B or A + BC BA + C Example 1: Fe(c) + Cu(NO3)2(aq) Fe(NO3)2(aq) + Cu(c) Example 2: 2Na(c) + 2H2O(l) 2NaOH(aq) + H2(g) Double Displacement The positive and negative ions of two compounds are interchanged compound + compound compound + compound A+X- + B+Y- A+Y- + B+XExample: 2KOH(aq) + H2SO4(aq) K2SO4 (aq) + H2O(l) Decomposition When energy in the form of heat, electricity, light, or mechanical shock is supplied, a compound may decompose to form simpler compounds and/or elements. The general form for this type of reaction: compound two or more substances AB A + B Example: 2KClO3(c) 2KCl(c) + 3O2(g) Synthesis In a synthesis reaction two or more simple substances (compounds and/or elements) are combined to form one new and more complex substance. The general form is : Element + element compound or compound + compound compound A + X AX Example 1: 2Na(c) + Cl2(g) 2NaCl(c) Example 2: SO2(g) + H2O(1) H2SO3(aq) PRACTICE PREDICTING PRODUCTS: For each of the following word equations identify the type or rxn, predict the products(s), write the complete skeleton equation, and balance the equation. 1.) silver nitrate + zinc chloride ________________________________________________ Type of rxn: ____________________________________ Skeleton equation: AgNO3 + ZnCl2 __________________________________________ Balanced equation: ______________________________________________________________ ---------------------------------------------------------------------------------------------------------------------------2.) zinc + hydrochloric acid _____________________________________________________ Type of rxn: _____________________________________ Skeleton equation: Zn + HCl ___________________________________________________ Balanced equation: _______________________________________________________________ ---------------------------------------------------------------------------------------------------------------------------3.) aluminum + oxygen _________________________________________________________ Type of rxn: ______________________________________ Skeleton equation: Al + O2 ___________________________________________________ Balanced equation: ______________________________________________________________ ---------------------------------------------------------------------------------------------------------------------------4.) HgO _____________________________________________________________________ Type of rxn: _______________________________________ Skeleton equation: _______________________________________________________________ Balanced equation: ______________________________________________________________ WAVES Scientists have observed that many elements emit (give off) visible light when they are heated in a flame. The light that each element emits is related to the arrangement of the electrons in its atoms. This light is a type of electromagnetic radiation (energy) that acts like a wave. There are different types of waves, some visible to us and others that we cannot see. Below is the electromagnetic spectrum, which arranges all these waves according to their wavelengths and frequencies: All waves have similar characteristics and components: Visible light Wavelength Wavelength is the distance between two consecutive troughs or two consecutive crests. The symbol used for wavelength is the Greek letter lambda (λ). It is usually measured in meters (m), centimeters (cm), or nanometers (nm, 1 nm = 1 x 10-9 m). Frequency (represented by ν, the Greek letter nu) is the number of wavelengths that pass a given point per second. The unit for frequency is the Hertz (Hz, waves per second). Different waves have different wavelengths and frequencies. The following are the relationships between wavelength and frequency: - As the wavelength increases, the frequency decreases As the wavelength decreases, the frequency increases Light, emitted by atoms of elements, is a form of energy. This energy is related to the wavelength and frequency of the light emitted through the following formulas: c=λxν - c is the speed of light (3.00 x 108 meters / second) - λ is the wavelength of the wave - ν is the frequency of the wave Ephoton = h x ν - Ephoton is energy - h is Planck’s constant (6.626 x 10-34 J s) - ν is the frequency of the wave Using the previous information, solve the following problems: 1. What is the frequency of green light, which has a wavelength of 4.90 x 10-7 m? 2. An X-ray has a wavelength of 1.15 x 10-10 m. What is its frequency? 3. A popular radio station broadcasts with a frequency of 94.7 MHz. What is the wavelength of the broadcast? (1 MHz = 106 Hz) 4. Tiny water drops in the air disperse the white light of the sun into a rainbow. What is the energy of a photon from the violet portion of the rainbow if it has a frequency of 7.23 x 1014 Hz? 5. What is the energy of each of the following types of radiation and what type of radiation is it (use the electromagnetic spectrum to determine the type of radiation)? a. 6.32 x 1020 Hz b. 9.50 x 1013 Hz c. 1.05 x 1016 Hz