Heat is the thermal energy transferred from one object to
advertisement

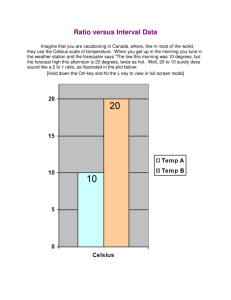
Matter contains thermal energy, not heat. Heat is the thermal energy in transit. Heat is the thermal energy transferred from one object to another due to a temperature difference. Heat is the to the total kinetic energy of the atoms or molecules. Heat is measured in joules When you strike a nail with a hammer, it becomes warm. Why? When you put a flame to a liquid, the liquid becomes warmer as its molecules move faster. Why? Temperature, the degree of “hotness” or “coldness” of an object. Temperature is the to the average (NOT total) kinetic energy of the atoms or molecules making it up. - The scale most often used world-wide is the Celsius thermometer, where a zero (0) is assigned to the temperature at which water freezes, and 100 is assigned to the temperature at which water boils (at standard atmospheric pressure). - In the U.S., the number 32 is traditionally assigned to the temperature at which water freezes, and the number 212 is the temperature at which water boils. This is called the Fahrenheit scale. - The absolute temperature scale is called the Kelvin scale. Absolute zero is 0 K. The melting point of ice is 273 K, and the boiling point of water is 373 K. There are no negative numbers on the Kelvin scale. - Here’s the part you all LOVE to hate: -How to convert from one scale to the other. Fahrenheit to Celsius Celsius to Kelvin Celsius to Fahrenheit Kelvin to Celsius Here's a trick for converting Celsius to Fahrenheit in your head: 1) double the Celsius temperature 2) subtract one tenth of this value 3) add 32 EXAMPLE: let's use 30 degrees C as an example. 1) double the Celsius temperature (2 x 30 = 60) 2) subtract one tenth of this value (60 - 6 = 54) 3) add 32 (54 + 32 = 86 degrees F) - In contrast to high temperatures, there is a definite limit at the opposite end of the scale, called absolute zero. Heat capacity as the amount of energy needed to change the temperature of a material by one degree. Sample Problem How much heat is needed to raise the temperature of 30 kg of liquid water from 10 0C to 20 0C ? Q = mcΔ T = 30 Kg x 4.19 J/kg 0C x (20-10)0C = 1,257J Sample Problem A 2.0-kg aluminum pan is heated on the stove from 20°C to 110°C. How much heat had to be transferred to the aluminum? The specific heat capacity of aluminum is 900 J/kg°C. Q = cmΔT Q = (900 J/kg°C ) (2.0 kg) (110°C - 20°C) Q = 162,000 J Q = 1.62 x 105 J Evaporation Melting Condensation Freezing Latent Heat is the amount of energy needed to change the state of a mater at constant temperature. Heat is a form of energy, and it is measured in joules. - A unit of heat common in the U.S. is the calorie, which is defined as the amount of heat energy needed to change the temperature of 1 gram of water by 1 Celsius degree 1 calorie = 4.18 joules - Conduction involves the transfer of heat through direct contact - Heat conductors conduct heat well, insulators do not Heat Transfer: Convection - Takes place in liquids and gases as molecules move in currents - Heat rises and cold settles to the bottom Heat Transfer: Radiation -Heat is transferred through space -Energy from the sun being transferred to the Earth Which picture shows the object that has more kinetic energy of particles? Figure A Figure B Emitters and absorbers • The Sun gives out the heat. • It is known as an emitter / radiator • The Earth takes in the heat. • It is known as an absorber. Good and Bad Emitters/Absorbers • A poor emitter would be a poor absorber. • A good emitter would also be a good absorber. Good emitter/absorber Poor emitter/absorber Dull, black surface Shiny, silver surface Rough surface Smooth surface

![Temperature Notes [9/22/2015]](http://s3.studylib.net/store/data/006907012_1-3fc2d93efdacd086a05519765259a482-300x300.png)