Electron configuration exceptions
advertisement

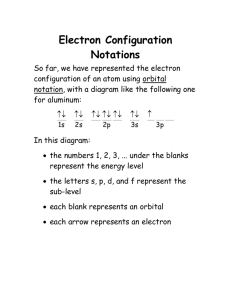
Electron Configuration cont’d MS. MCGRATH CHEMISTRY 11 Aufbau principle • States that in going from a hydrogen atom to a larger atom, you add protons to the nucleus and electrons to orbitals • You start at the orbitals having the lowest energy level and fill them, in order of increasing energy, until you run out of electrons Pauli exclusion principle • States that an orbital can be empty, have one electron, or at most have two electrons Hund’s rule • States that electrons in the same sublevel will not pair up (occupy the same orbital) until all the orbitals in the sublevel have been at least half filled Electron configurations •The lanthanides are the 14 elements removed from period 6 of the periodic table • The actinides are the 14 elements removed from period 7 of the periodic table • The above two groups do have unique electron configurations Electronic configuration - Lanthanides Lanthanum, atomic number 57: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 or shorthand notation: [Xe]6s2 5d1 Electronic configuration - Lanthanides Erbium, atomic number 68: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f11 or shorthand notation: [Xe]6s2 5d1 4f11 Electronic configuration - Lanthanides Hafium, atomic number 72: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d1 or shorthand notation: [Xe]6s2 5d1 4f14 5d1 Electronic configuration - Lanthanides Note: We place one electron in 5d then we fill up 4f with fourteen electrons then return to 5d and place the remaining nine electrons Electron configuration - Actinides Now you try: Radium, atomic number 88 Uranium, atomic number 92 Rutherfordium, atomic number 104 Electron configuration - Actinides Radium, atomic number 88: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d9 6p6 7s2 or shorthand notation: [Rn]7s2 Electron configuration - Actinides Uranium, atomic number 92: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d9 6p6 7s2 6d1 5f3 or shorthand notation: [Rn]7s2 6d1 5f3 Electron configuration - Actinides Rutherfordium, atomic 104: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 5d1 4f14 5d9 6p6 7s2 6d1 5f14 6d1 or shorthand notation: [Rn]7s2 6d1 5f14 6d1 Electron configuration - Actinides Note: We place one electron in 6d then we fill up 5f with fourteen electrons then return to 6d and place the remaining nine electrons Electron configuration - exceptions Chromium and Copper See video and make notes Electron configuration for ions Watch video and copy notes Electron configuration for ions Practice