Phase Equilibria
advertisement
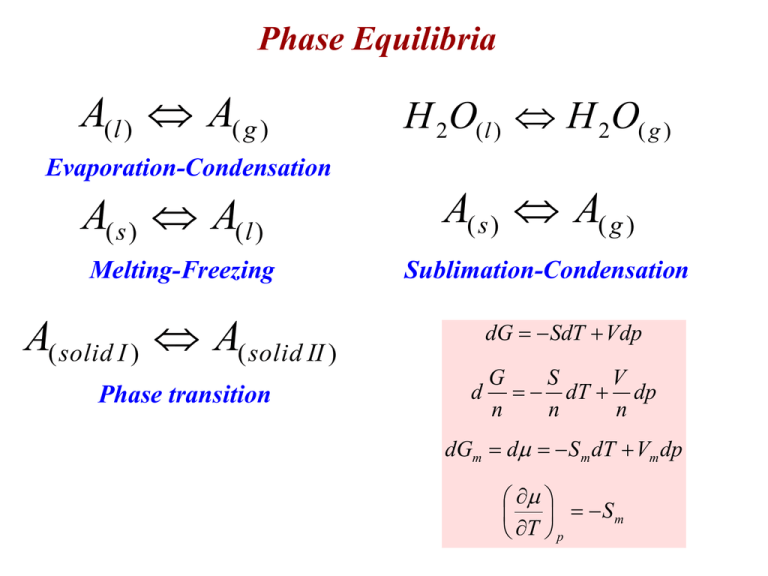
Phase Equilibria A( l ) A( g ) Evaporation-Condensation A( s ) A(l ) Melting-Freezing H 2O( l ) H 2O( g ) A( s ) A( g ) Sublimation-Condensation A( solid I ) A( solid II ) Phase transition dG SdT Vdp d G S V dT dp n n n dGm d S m dT Vm dp Sm T p Sg >> Sl > Ss solid solid Sm T p liquid S mliquid T p gas S mgas T p Sm slope in T diagram The most stable phase is that with lowest chemical potential. dGm d S m dT Vm dp Pressure Effect solid Vmsolid p T liquid Vmliquid p T gas Vmgas p T Vmgas Vmliquid or Vmsolid usually Vmliquid Vmsolid -T curve of gases much more largely affected by pressure change than liquids or solids few subs tan ces : Vmsolid Vmliquid Pressure increase: - Boiling point elevation - Freezing point elevation/depression Phase Diagram CO2 Clapeyron Equation A( l ) A( g ) A( s ) A( g ) A( s ) A(l ) A( ) A( ) At Equilibrium d d S m dT Vm dp S m dT Vm dp V V m m V dp S m m S m Vtr dp S tr dT Clapeyron Equation dT Vm dp S m S m dT dp S tr dT Vtr Solid-Liquid Equilibrium A( s ) A(l ) dp Str S fus dT Vtr V fus S l m S dp s m S H fus V fusTm fus H fus Tm dT p2 p1 Slope of pT-curve H fus dp dT V fusTm p2 H fus p1 fus dp V H fus V fus Tm' ln Tm Tm' p2 dT T Tm p1 m Usually positive ~ 40 atm/K 40 atm are needed to change the melting point by 1 K V fus Vml Vms usually , Vml Vms V fus 0 some systems, Vml Vms p2 p1 Upon pressure increase p2 p1 H fus V fus V fus 0 Tm' ln Tm p2 p1 0 if V fus 0 Tm' ln 0 Tm Tm' Tm Melting point elevation if V fus 0 Tm' ln 0 Tm Tm' Tm Melting point depression water Ice skating p2 p1 H fus V fus p2 p1 Tm' H fus Tm Tm' Tm ln ln Tm V fus Tm H fus V fus ln 1 x x Tm' Tm ln 1 Tm if x is very small H fus Tm' Tm p2 p1 V fus Tm H fus Tm p V fus Tm if pressure is changed by p, the melting point will change by Tm Liquid-Gas Equilibrium A( l ) A( g ) S vap H vap Tb dp S vap dT Vvap dp H vap p dT T RT Slope of pT-curve always positive ~ 0.04 atm/K Boiling point increases by 25 K upon increasing the pressure by 1 atm. applies also to s-g equilibrium Vtr Vvap Vmg Vml Vmg 1000 Vml V g m V l m V g m RT p dp H vap dT 2 p R T T p dp H vap b 2 dT p p R T p T 2 1 b 1 p2 H vap 1 p2 1 ln p1 R Tb p2 Tb p1 H vap 1 1 p T ln v 2 pv T1 R T2 T1 Clausius-Clapeyron Equation dp H vap dT p R T2 H vap 1 ln p C R T p eC e H vap 1 R T p const e at T Tb H vap 1 R T pv patm Determine the change in the freezing point of ice upon pressure increase from 1 atm to 2 atm. Vm(water)=18.02 cm3/mol and Vm(ice)=19.63 cm3/mol at 273.15 K. Hfus=6.009 kJ/mol. Benzene has a normal boiling point of 353.25 K. If benzene is to be boiled at 30oC, to what value must the pressure be lowered. Hvap=30.76 kJ/mol Phase Rule F: Number of degrees of freedom Number of independent variables that can be changed without changing the number of phases C: number of independent components P: number of coexisting phases F=1 F=2 F=0 F=1 Liquid-Gas Equilibrium of a binary mixture Ideal solution: H mix 0 Vmix 0 A B A B mixture H mix 0 Vmix 0 Energy of interaction AA,BB = A-B Intramolecular forces AA,BB = A-B 100 mL A 100 mL B 200 mL mixture Vm Apure Vm B pure Vm Amixture Vm Bmixture Ideal solutions obey Raoults Law pi xi p o i pi xi p o i V V L (pA)solvent > L (pA)solution nA xA 1 n A nB psolution p A p B psolution x A p xB p o A p-x phase diagram o B T=const. psolution 1 xB p Ao xB p Bo psolution p xB p xB p o A o A o B P Total PA PB X APA X BPB psolution p Ao p Bo p Ao xB y a b psolution pA pB x PA X A PA PB X BPB y B p Ao xB o pB p Ao pBo y B V substitute in ptotal p Ao pBo p Ao xB L pBo p Ao ptotal o pB p Ao pBo y B A+B L gas ptotal p A pB p A xVA ptot p B xVB ptot p A y A ptot p B y B ptot pB xBL p Bo x A p Ao yB ptot x AL p Ao xBL p Bo x A p Ao xB p Bo xB p Bo yB o p A p Bo p Ao xB solve for xB V T const. Ex. Benzene and Toluene • Consider a mixture of benzene, C6H6, and toluene, C7H8, containing 1.0 mol benzene and 2.0 mol toluene. At 20 °C, the vapor pressures of the pure substances are: P°benzene = 75 torr P°toluene = 22 torr • Assuming the mixture obeys Raoult’s law, what is the total pressure above this solution? 23 T-x phase diagram p=const. Lever Rule n L xBa xBc nV xBc ' xBa n L nV ntot n A nB ntot Distillation p=const. Colligative Properties Colligative Properties Kf and Kb 31 Ex. Boiling Point Elevation A 2.00 g sample of a large biomolecule was dissolved in 15.0 g of CCl4. The boiling point of this solution was determined to be 77.85 °C. Calculate the molar mass of the biomolecule. For CCl4, the Kb = 5.07 °C/m and BPCCl4 = 76.50 °C. Tb K b msolute nsolute msolute wtsolvent / kg nsolute Tb K b wtsolvent / kg Tb wtsolvent / kg nsolute Kb Tb T T o 77.85o C 76.50o C 1.35o C wtsolvent / kg 0.015 kg K b 5.07 o C / m nsolute 4.026 10 3 mol Mwt solute msolute 2g 497 g / mol 3 nsolute 4.026 10 mol Ex. Freezing Point Depression Estimate the freezing point of a permanent type of antifreeze solution made up of 100.0 g ethylene glycol, C2H6O2, (MM = 62.07) and 100.0 g H2O (MM = 18.02). T f K f msolute msolute mEG nEG wt EG / kg nEG mEG 100 g 1.611 mol Mwt EG 62.07 g / mol nEG 1.611 T f K f 1.86 30o C wt EG / kg 0.10 T f T fo T f T f T fo T f 0o C 30o C 30o C 33 Membranes and Permeability Membranes – Separators – Example: Cell walls – Keep mixtures organized and separated Permeability – Ability to pass substances through membrane Semipermeable Membrane – Some substances pass, others don’t. – Selective Osmosis and Osmotic Pressure A. Initially, Soln B separated from pure water, A, by osmotic membrane (permeable to water). No osmosis occurred yet B. After a while, volume of fluid in tube higher. Osmosis has occurred. 35 Osmotic pressure (p): Pressure needed to stop the flow. Flow of water molecules Net flow Column rises Pressure increases Increase of flow from right to left Finally: Equilibrium established Flow of water molecules Net flow = 0 Equation for Osmotic Pressure • Assumes dilute solutions p=iMRT – p = osmotic pressure – i = number of ions per formula unit = 1 for molecules – M = molarity of solution • Molality, m, would be better, but M simplifies • Especially for dilute solutions, where m M – T = Kelvin Temperature – R = Ideal Gas constant = 0.082057 L·atm·mol1K1 37 Eye drops must be at the same osmotic pressure as the human eye to prevent water from moving into or out of the eye. A commercial eye drop solution is 0.327 M in electrolyte particles. What is the osmotic pressure in the human eye at 25°C? p = MRT T(K) = 25°C + 273.15 L atm p 0.327 M 0.08206 298 K 8.00atm K mol Using p to determine MM The osmotic pressure of an aqueous solution of certain protein was measured to determine its molar mass. The solution contained 3.50 mg of protein in sufficient H2O to form 5.00 mL of solution. The measured osmotic pressure of this solution was 1.54 torr at 25 °C. Calculate the molar mass of the protein. 1atm 1.54torr p mol 760torr M 8.28 10 5 L atm RT L 0.08206 298K K mol n M V 8.28 105 M 5.00 103 L 4.14 107 mol mass 3.50 10 3 g 3 Mwt 8 . 45 10 g / mol 7 n 4.14 10 mol