S4 Chemistry Review Sheet – Unit 1 Atomic structure
advertisement

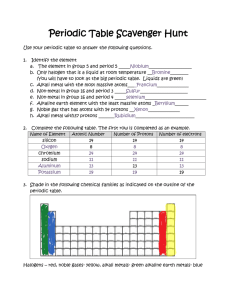
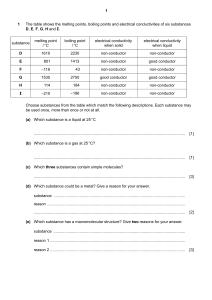
Name: Date: Teacher: S4 Chemistry Review Sheet – Unit 1 Atomic structure During S1-S3 the following learning outcomes were covered: o I can state that everything in the world is made up of atoms. o I can describe a substance as an element, if it contains only one kind of atom, or a compound, if it is formed when atoms of two or more different elements are chemically joined. o I can state that elements are arranged in the periodic table, each with its own unique symbol. o I can state that elements with similar chemical properties are placed together in vertical groups. o I can state that elements are arranged in the periodic table in order of increasing atomic number. o I can categorise elements as metals and non-metals, depending on their electrical conductivity. Noting that carbon in the form of graphite is an exception. The following tasks are designed to test how confident you are with this learning. Complete the tasks to the best of your ability. Task 1-Gathering information Use the periodic table in your data book to complete the information below. Element name Gold Symbol Atomic number Au Fe 26 7 Oxygen 2 Si Task 2-Applying knowledge Look at the particle pictures below and label each box to show whether it is an element or compound. Task 3-Reading for information Below are descriptions of three different elements. Read the descriptions and then use your periodic table to work out which element is being described. Element 1 I am an alkali metal. Like all the alkali metals I am very reactive. I am so reactive, in fact, that I must be stored under oil to stop me from reacting with air and water in the atmosphere. I am more reactive than sodium but less reactive than rubidium. Who am I? Element 2 I am a halogen. Like all halogens I am found in group 7 of the periodic table and react with metal elements to make salts. I am unusual because I am the only non-metal element that is a liguid at room temperature. I am more reactive than iodine but less reactive than chlorine. Who am I? Element 3 I am a noble gas. Like all noble gases I am very unreactive. I do have one interesting feature-I glow with a red coloured light when electricity is passed through me and so I am often used in sign writing for shops and businesses. My atoms are bigger than helium but smaller than argon. Who am I? Task 4-Making generalisations The circuit shown opposite is used to test the conductivity of different elements. If the bulb lights the element is a conductor and if the bulb does not light the element is a non-conductor. The results of the experiment are shown in the table below. Element copper iron aluminium zinc sulphur iodine silicon carbon (diamond) carbon (graphite) Metal or non-metal metal metal metal metal non-metal non-metal non-metal non-metal non-metal Conductivity conductor conductor conductor conductor non-conductor non-conductor non-conductor non-conductor conductor 1. Use the results in the results table to write a general statement describing the conductivity of elements. 2. Which element does not fit this general statement? Now that you have tried all the tasks either hand in to your teacher for marking or use the mark scheme available to check your work. After you have checked your answers, highlight the learning outcomes below (pink if you feel your answers to the tasks show that you are confident with the learning outcome and green if you feel you still need more support). o I can state that everything in the world is made up of atoms. o I can describe a substance as an element, if it contains only one kind of atom, or a compound, if it is formed when atoms of two or more different elements are chemically joined. o I can state that elements are arranged in the periodic table, each with its own unique symbol. o I can state that elements with similar chemical properties are placed together in vertical groups. o I can state that elements are arranged in the periodic table in order of increasing atomic number. o I can categorise elements as metals and non-metals, depending on their electrical conductivity. Noting that carbon in the form of graphite is an exception. Now try to identify some next steps (a few suggestions have been made below but this list is not exhaustive!) Next steps: Log into www.evans2chemweb.co.uk using the username: dunbar and password: atoms. There is a virtual lab, where you can carry out a conductivity testing experiment. Start to make a set of Chemistry flash cards or a Chemistry dictionary. Write the word element on one side and write the definition of an element on the other. Do the same for the word compound. You can add more words to your cards/dictionary as you learn more new words. Use the internet to find an interactive periodic table and use it to carry out your own research into a few of the elements. Try to find out the symbol, atomic number, which group the element belongs to and the element’s chemical and physical properties and how this is related to its use. Practise making generalisations using the following past paper questions available on www.sqa.org.uk . SG Credit 2007Q11b)i), SG Credit 2008Q18d), SG Credit 2011Q12b)i)