Charles's Law - richardkesslerhfa
advertisement

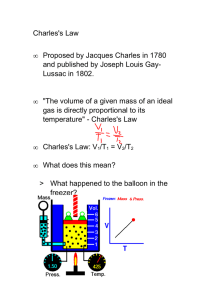
CHARLES’S LAW The Second Gas Law Objectives • Upon completion of this presentation, you will be able to • describe the relationship between the volume and the temperature of an ideal gas • use Charles’s law to calculate the final volume of a gas when given the initial temperature and volume and the final temperature • use Charles’s law to calculate the final temperature of a gas when given the initial temperature and volume and the final volume Introduction • In the 1780’s, Jacques Charles was investigating the properties of gases. • He found a relationship between volume, V, and temperature, T, when the pressure, P, is held constant. • Volume is directly related to temperature. • As the temperature increases, the volume increases. • As the temperature decreases, the volume decreases. Introduction • This behavior would be expected from the assumptions of the kinetic theory. • As the temperature increases, the average speed of the gas particles also increases. • This causes the collisions with the walls of the container to be more forceful. • More force over the same area gives more pressure. • To keep the pressure constant, we would need to increase the volume of the container. Introduction • We can write Charles’s law two different ways: • V/T = k or V = kT • where "k" is a constant. • V1/T1 = V2/T2 • where V1 and T1 are initial volume and temperature • where V2 and T2 are final volume and temperature Introduction • Charles stated his law using the first notation, V = kT. • The change in volume of a gas is directly proportional to the temperature when the pressure is held constant. •V∝T ⇒ V = kT • We most often use the second notation, V1/T1 = V2/T2, to solve problems. Application • When we are trying to solve a Charles's law problem, we will need to know three of the four variables. • For V1/T1 = V2/T2 we can solve for: V1 = V2T1 T2 T1 = T2V1 V2 V2 = V1T2 T1 T2 = T1V2 V1 Example 1 – Finding V2 What is the final volume, V2, of 2.00 L of helium gas at an initial temperature of 300K, and a final temperature of 500 K? V1 = 2.00 L T1 = 300 K V2 = ? L T2 = 500 K V2 = V1T2 = T1 (2.00 L)(500 K) 300 K = 3.33 L Sample Problems – Finding V2 1. What is the final volume of hydrogen gas with an initial volume of 3.00 L, an initial temperature of 300 K, and a final temperature of 400 K? V2 = 4.00 L 2. What is the final volume of oxygen gas with an initial volume of 0.120 L, an initial temperature of 500 K, and a final temperature of 200 K? V2 = 0.0480 L 3. What is the final volume of chlorine gas with an initial volume of 2.40 L, an initial temperature of 200 K, and a final temperature of 250 K? V2 = 3.00 L 4. What is the final volume of carbon monoxide gas with an initial volume of 0.0480 L, an initial temperature of 273 K, and a final temperature of 625 K? V2 = 0.110 L Example 2 – Finding T2 What is the final temperature, T2, of argon gas at an initial volume of 4.20 L, an initial temperature of 250 K, and a final volume of 4.80 L? V1 = 4.20 L T1 = 250 K V2 = 4.80 L T2 = ? L T2 = T1V2 = V1 (250 K)(4.80 L) = 285 K 4.20 L Sample Problems – Finding T2 1. What is the final temperature of neon gas with an initial volume of 4.00 L, an initial temperature of 350 K, and a final volume of 0.100 L? T2 = 8.75 K 2. What is the final temperature of helium gas with an initial volume of 1.20 L, an initial temperature of 250 K, and a final volume of 0.600 L? T2 = 125 K 3. What is the final temperature of argon gas with an initial volume of 480 L, an initial temperature of 300 K, and a final volume of 1,200 L? T2 = 750 K 4. What is the final temperature of fluorine gas with an initial volume of 40.4 L, an initial temperature of 280 K, and a final volume of 542 L? T2 = 3,760 K Example 3 – Finding V1 What was the initial volume, V1, of neon gas that had an initial temperature of 200 K, a final volume of 0.500 L, and a final temperature of 500 K? V1 = ? L T1 = 200 K V2 = 0.500 L T2 = 500 K V1 = V2T1 (0.500 L)(200 K) = = 0.200 L T2 500 K Sample Problems – Finding V1 1. What is the initial volume of nitrogen gas with an initial temperature of 600 K, a final temperature of 300 K, and a final volume of 0.100 L? V1 = 0.200 L 2. What is the initial volume of oxygen gas with an initial temperature of 250 K, a final temperature of 300 K, and a final volume of 6.00 L? V1 = 5.00 L 3. What is the initial volume of chlorine gas with an initial temperature of 1,200 K, a final temperature of 400 K, and a final volume of 3,500 L? V1 = 10,500 L 4. What is the initial volume of fluorine gas with an initial temperature of 294 K, a final temperature of 742 K, and a final volume of 85.9 L? V1 = 34.0 L Example 4 – Finding T1 What was the initial temperature, T1, of argon gas at an initial volume of 0.100 L, a final volume of 0.125 L, and a final temperature of 298 K? V1 = 0.100 L T1 = ? L V2 = 0.125 L T2 = 298 K T1 = T2V1 = V2 (298 K)(0.100 L) = 238 K 0.125 L Sample Problems – Finding T1 1. What is the initial temperature of radon gas with an initial volume of 4.00 L, a final volume of 2.00 L, and a final temperature of 250 K? T1 = 500 K 2. What is the initial temperature of neon gas with an initial volume of 12.0 L, a final volume of 15.0 L, and a final temperature of 400 K? T1 = 320 K 3. What is the initial temperature of helium gas with an initial volume of 0.720 L, a final volume of 0.600 L, and a final temperature of 350 K? T1 = 420 K 4. What is the initial temperature of ammonia gas with an initial volume of 0.235 L, a final volume of 0.123 L, and a final temperature of 275 K? T1 = 525 K Summary • Charles’s Law: • At a constant pressure, • the volume of a gas is directly proportional to its volume. • Equations: • V/T = k or V = kT, where k is a constant • V1/T1 = V2/T2