Chapter 21 Nuclear Chemistry

Chapter 21
Nuclear Chemistry
12 th edition
21.1 Radioactivity
Review from Ch. 2
Subatomic particles
Atoms are neutral: # protons = # electrons
Isotopes
– atoms with same number of protons but different number of neutrons
Mass #
Atomic #
A
Z
X
element symbol
Particle nucleon
Position Charge Mass (kg) electron outside nucleus −1 9.11 x 10 -31 proton inside nucleus neutron inside nucleus
+1
0
1.67 x 10 -27
1.67 x 10 -27
Nuclear Chemistry deals with only the nucleus.
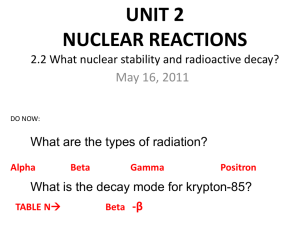
radioactivity – spontaneous emission of radiation
nuclide – nucleus with specified number of protons and neutrons radionuclide – radioactive nuclide radioisotopes – atoms containing radioactive nuclei radioactive decay – spontaneous decomposition to form a different, more stable, nucleus with the production of one or more particles decay series – multiple decay steps through which radioactive nuclides go to reach a stable state
Decay Series
Common Particles in Radioactive
Decay and Nuclear Reactions
Decay products: (See Tables 21.2 and 21.3, p. 879) alpha ( α) particle 4
2
He or 4
2
α Common for heavy radionuclide beta ( β) particle gamma ( positron g
) ray
0
-1
β or 0
-1 e High-speed electrons; atomic number increases when a decay product
0
0 g
High-energy photons; often accompanies other decay like electron capture
0
1
β or 0
1 e Atomic number decreases when a decay product
Decay reactants: electron capture 0
-1 e neutron capture 1
0 n
Addition of electron(s) with production of g rays
Neutron capture causes transmutations and fission-based chain reactions
Fission of
235
U initiated by neutron capture
Chain Fission Reaction
Nuclear Equations – sums of both mass and atomic numbers on both sides of equation are equal
Examples:
68
31
Ga + 0
-1 e → _____
212
87
Fr → 208
85
At + ______
263
106
Sg → ______ + 4
2
He
21.4 Rates of Radioactive Decay
Radioactive decay follows 1 st order kinetics.
All half-lives are equal.
ln
N
N
0 t
kt
N – # of radioactive nuclei k – decay constant t
1 / 2
0 .
693 k
Individual half-lives vary tremendously:
214 Po 2 x 10 -4 s
144 Nd 5 x 10 15 year
Half-lives
Practice exercise, p. 890
Practice problem 21.33
Practice problem, 90 Sr from 1 st atomic explosion
Mastering problem 21.41