week08.1.parenterals 2004
advertisement
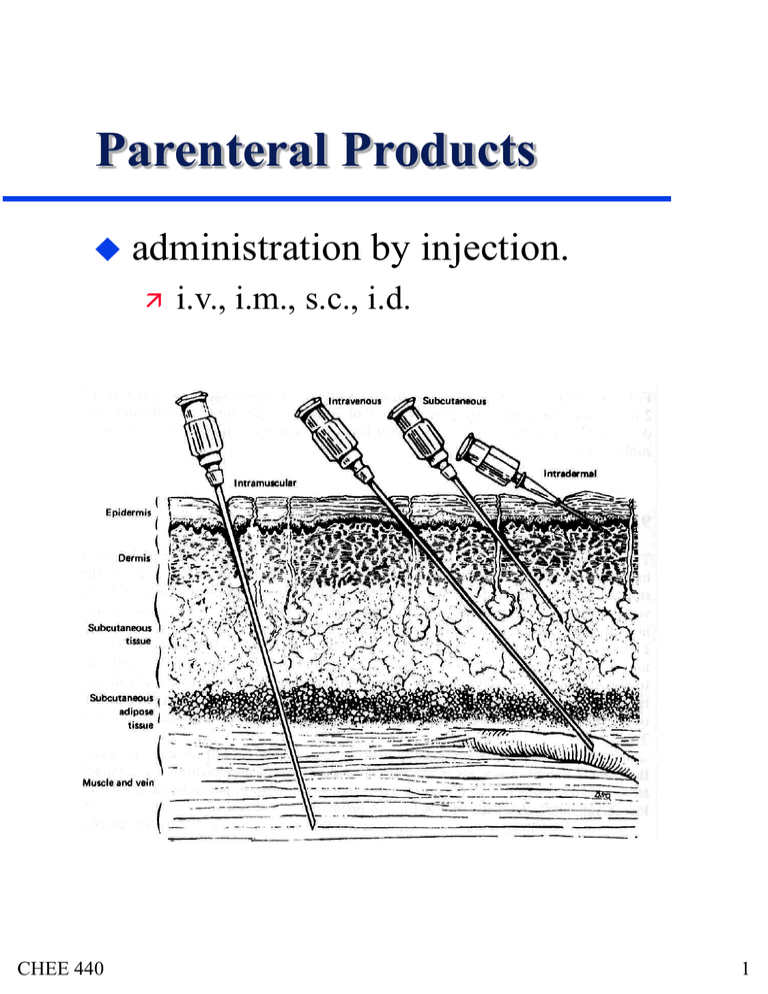
Parenteral Products administration CHEE 440 by injection. i.v., i.m., s.c., i.d. 1 Parenterals advantages rapid onset of action predictable, nearly complete bioavailability avoidance of problems associated with GIT reliable admin. to very ill and comatose disadvantages cost (professional care) patient compliance formulations CHEE 440 solutions suspensions emulsions colloidal dispersions 2 Solution Formulation solvents must meet purity standards restricted number and kind of added substances no coloring agents permitted products are : always sterilized pyrogen-free prepared in environmentally controlled areas under sanitary conditions volumes used are specific to application CHEE 440 3 Composition active agent anti-oxidants CHEE 440 e.g. ethanol, PEG, glycerin tonicity agents inactivate metals which catalyze degradation co-solvents e.g. citric acid, sodium phosphate, sodium acetate, dipotassium hydrogen phosphate chelating agents ex. ascorbic acid, sodium bisulfite buffers (pH) Need to consider solubility related to semi-permeable nature of cell membranes and osmotic pressure of solution preservatives 4 Preservatives for preps that can’t be sterilized easily required for multiple-dose formulations good growth media include » most aqueous preps (esp. syrups), emulsions, suspensions, creams preps which contain alcohol do not require sterilization or preservation Criteria CHEE 440 effective soluble sufficiently non-ionized in solution nonirritating, nonsensitizing, nontoxic chemically stable compatible with other ingredients 5 Preservatives Modes of Action modification of cell membrane permeability lysis and cytoplasmic leakage protein denaturation inhibition of cellular metabolism oxidation and/or hydrolysis of cellular components (enzymes) CHEE 440 antifungals » benzoic acid, parabens, sodium benzoate, sodium propionate antimicrobials » benzyl alcohol, phenol, chlorobutanol, cetylpryidinium chloride 6 Types of Water water source water typically contaminated purified by distillation or reverse osmosis not required to be sterile, but pyrogenfree intended to be used within 24 hours sterile CHEE 440 for injection water for injection as above but sterilized intended for use in reconstituting powders 7 Osmotic Pressure : Clinical Relevance whole blood, plasma, serum are complex mixtures of proteins, glucose, non-protein nitrogenous compounds, and electrolytes (Na, Ca, K, Mg, Cl, CO3 ) electrolytes determine osmotic pressure must formulate with osmotic pressure in mind is a colligative property CHEE 440 8 Boiling Point Elevation boiling pt of solution is higher than that of pure solvent consider a vapor in equilibrium with a solution at constant pressure RTb2 Tb X2 H v for very dilute solutions : RTb2 M1 Tb m2 K bm2 1000Hv » Kb = ebullioscopic constant (Tables) » Kb water = 0.51 K kg/mol CHEE 440 9 Freezing Point Depression assume solvent freezes as pure solvent RT f2 Tf X2 Hf RTf2 M1 Tf m 2 Kf m 2 1000Hf CHEE 440 Kf = cryoscopic constant (Tables) Kf water = 1.86 K kg/mol 10 Osmotic Pressure, P at t = 0 sucrose solution water semipermeable membrane at equilibrium h P gh CHEE 440 11 Osmotic Pressure, P water moves across membrane due to mL to R at equilibrium mw,R = mw,L nonideal solutions : RT P ln a 1 V1 ideal solutions : RT P ln X1 V1 ideally dilute solutions : P m2 RT CHEE 440 12 Electrolyte Solutions : Colligative Properties that P can be determined from Tb and Tf measurements note Van’t accounts for nonideality, increased number of moles produced ideally dilute CHEE 440 Hoff Factor, i P imRT 13 Tonicity extent of swelling or contraction of biological membrane (cells, mucous membranes) cell membranes are semipermeable hypertonic = higher P than cells causes cells to crenate or shrink hypotonic = lower P than cells causes cells to rupture (lyse) = same P (isoosmotic) isoosmotic doesn’t necessarily mean isotonic isotonic CHEE 440 14 Methods of Adjusting Tonicity Tf blood and tears = - 0.52˚C add appropriate amount of compound (ex. NaCl) to drug solution or add water to drug solution NaCl Equivalent Method E = amount of NaCl equivalent in P to 1 g of drug NaCl (w/v%) = 0.90 - E*[drug] (w/v%) values for E found in Tables (p 622-7 Remington) CHEE 440 15 Methods of Adjusting Tonicity White-Vincent Method (USP Method) calculates volume (V) in ml of isotonic solution that can be prepared by mixing drug with water/isotonic buffered solution V = w * E *111.1 w = wt. of drug (g) CHEE 440 16 Methods of Adjusting Tonicity Freezing Point Depression freezing point depressions of 1w/v% drug solutions (Tf1%) have been tabulated (p 622-627 Remington) choose appropriate solute for adjusting tonicity » using Tf,ref1% determine required amount (wref) to cover remaining Tf w ref 0.52 CTf1% Vreq 1% Tf, ref » Vreq = volume of water required » C = drug concentration (w/v%) CHEE 440 17 Example : 1. Make a 25 ml isotonic solution of 2.5 w/v % epinephrine bitartrate. 2. Do the same but now add 0.5w/v % phenol. CHEE 440 18 Containers CHEE 440 19 Freeze Drying used to dry heat-sensitive materials liquid P solid vapor T CHEE 440 20 Freeze Drying advantages degradation of product is minimized light, porous product no concentration of product during drying disadvantages product is very hygroscopic slow and expensive process CHEE 440 21