Parenteral nutrition 2013
advertisement

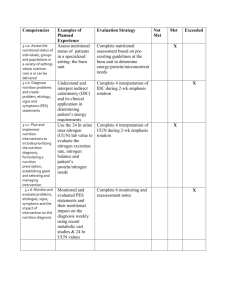
PARENTERAL NUTRITION (PN) Pharmacotherapy 4 for PharmD Spring 2013 References • • 1) 2) • • DiPiro:Parenteral Nutrition UpToDate: Nutrition support in critically ill patients: An overview Nutrition support in critically ill patients: Parenteral nutrition Koda-Kimble: Adult Parenteral Nutrition ESPEN Guidelines on Parenteral Nutrition: Intensive care. 2009. espen.info/documents/0909/Intensive%20Care.pdf Introduction Pharmacist's role in nutrition-support care requires knowledge of: • principles of patient selection, • initial therapy design, • preparation & dispensing of nutritional formulations, • outcome monitoring. Desired Outcomes • 4 steps to providing optimal care: 1.definition of nutrition goals 2.determination of nutrient requirements to achieve goals 3.delivery of required nutrients 4.assessment of nutrition regimen. Nutrition support goals • • • • correction of caloric & nitrogen imbalances, any fluid or electrolyte abnormalities, known vitamin or trace element abnormalities. Additionally: to ↓ metabolic response to injury by minimizing oxidant stress & favorably modulating immune response – without causing or worsening other metabolic complications. Specific caloric goals (a) adequate energy intake to promote normal growth & development in children, (b) energy equilibrium & preservation of fat calorie stores in well-nourished adults, (c) positive energy balance in malnourished patients with depleted endogenous fat stores. • Obese patients with excess endogenous fat stores (> 120% of IBW) may require less caloric support • Specific nitrogen goals are positive nitrogen balance or nitrogen equilibrium & improvement in serum concentration of visceral protein markers such as transferrin or prealbumin. Desired Outcomes (cont’d) • Who will benefit form PN? - patients who have nonfunctional GI tracts or are otherwise not candidates for enteral nutrition. • Routine monitoring: to ensure that nutrition regimen is suitable as patient's clinical condition changes & to minimize or treat complications early. • Potential risks of initiating therapy: infection & other metabolic abnormalities Estimation of Energy Expenditure Basal Energy Expenditure (BEE) (=BMR, basal metabolic rate) • Harris-Benedict Equations BEE men (kcal/day)=66.47 + 13.75 W + 5.0 H – 6.76 A BEE women (kcal/day)=655.10 + 9.56 W + 1.85 H – 4.68 A, or 20–25 kcal/kg/day (A, age in years; H, height in cm; W, weight in kg). Multiply the BEE by an adjustment factor (stress factor), depending on the level of metabolic stress and nutritional goal. In patients who are anabolic, hypermetabolic, critically ill, or are malnourished and/or have a fistula, use a stress factor of about 1.3– 1.5. Energy Requirements • Hospitalized patient, mild stress 20–25 kcal/kg/day • Moderate stress, malnourished 25–30 kcal/kg/day • Severe stress, critically ill 30–35 kcal/kg/day The Subjective Global Assessment Normal or Mild Malnutrition: • No change in dietary intake or inadequate dietary intake <2 weeks; and/or • No history or <2-day history of anorexia, nausea, vomiting, or diarrhea; and/or • Weight loss of <5% of usual body weight (UBW). Moderate Malnutrition: • Inadequate dietary intake 2 weeks; and/or • History of anorexia, vomiting, or diarrhea 1–2 weeks; and/or • Weight change of 5–9% of UBW over <6 months. Severe Malnutrition: • Inadequate dietary intake for 1 month; and/or • History of anorexia, vomiting, or diarrhea for 2 weeks; and/or • Visual evidence of wasting; and/or • Loss of 10% of UBW over <6 months. It has also been suggested that an unintentional weight loss of >10% over a 6month period indicates severe malnutrition. NB: if actual weight is less than his IBW, use actual (admission) weight. Indications for Adult PN 1. Inability to absorb nutrients via GIT because of 1 or > of the following: a. Massive small bowel resection b. Intractable vomiting when adequate EN is not expected for 7–14 days. c. Severe diarrhea d. Bowel obstruction e. GI fistulae: patients with prolonged inadequate nutritional intake > 5–7 days who are not candidates for EN EN, enteral nutrition; GI, gastrointestinal; HSCT, hematopoietic stem cell transplantation; PN, parenteral nutrition; SBS, short-bowel syndrome. Indications for Adult PN (cont’d) 2. Cancer: antineoplastic therapy, radiation therapy, or HSCT • may be used in moderately to severely malnourished patients receiving active anticancer treatment who are not candidates for EN • is not routinely indicated for well-nourished or mildly malnourished patients undergoing surgery, chemotherapy, or radiation therapy • is unlikely to benefit patients with advanced cancer whose malignancy is unresponsive to treatment (may be appropriate for some carefully selected patients with estimated life expectancy of > 40–60 days & strong social & financial support) • is appropriate for patients undergoing HSCT who are malnourished & who are anticipated to be unable to ingest &/or absorb adequate nutrients for 7–14 days. Indications for Adult PN (cont’d) 3. Pancreatitis: severe pancreatitis with prolonged inadequate nutritional intake longer than 5–7 days who are not candidates for EN (if EN exacerbates abdominal pain, ascites, or fistula output) 4. Critical Care a. in patients in whom EN is contraindicated or is unlikely to provide adequate nutritional requirements within 5–10 days b. organ failure (liver, renal, or respiratory): patients with moderate to severe catabolism when EN is contraindicated c. burns: in patients in whom EN is contraindicated or is unlikely to provide adequate nutritional requirements within 4–5 days ESPEN guidelines: • All ICU patients who are not expected to be on normal nutrition within 3 days should receive PN within 24 to 48 h if EN is contraindicated or if they cannot tolerate EN. Indications for Adult PN (cont’d) 5. Perioperative PN a. Preoperative: for 7–14 days for patients with moderate to severe malnutrition who are undergoing major GI surgery, if operation can be safely postponed b. Postoperative: in patients in whom EN is contraindicated or is unlikely to provide adequate nutritional requirements within 7–10 days 6. Hyperemesis gravidarum: when EN is not tolerated 7. Eating disorders: PN should be considered for patients with anorexia nervosa & severe malnutrition who are unable or unwilling to ingest adequate nutrition Indications for Pediatric PN 1. When EN is unlikely to provide adequate nutritional requirements a. Premature infant within 24–48 hours b. Other pediatric patients within 5–7 days 2. When GIT is not functional or cannot be assessed a. Massive small bowel resection resulting in short-bowel syndrome b. Neonatal necrotizing enterocolitis c. Severe inflammatory bowel disease d. Intractable diarrhea &/or vomiting e. Graft-versus-host disease f. Postchemotherapy 3. Infants & children requiring extracorporeal membrane oxygenation 4. Organ failure (liver, renal, pulmonary, pancreas) when EN is contraindicated & child is catabolic Contraindications to PN (UpToDate) • • • • • • hyperosmolality, severe hyperglycemia, severe electrolyte abnormalities, volume overload, inadequate attempts to feed enterally. Sepsis or SIRS is a relative contraindication to parenteral nutrition Timing of PN • Adult PN therapy is not emergent intervention & should not be initiated until patient is hemodynamically stable. • In general, adults who are not candidates for EN should be considered candidates for PN after 7-14 days of suboptimal nutritional intake. • Children: early PN within the first 24 hours of life for infants with birth weight < 1,500 grams. • - within 5 to 7 days in other pediatric patients who are unable to meet their nutrient requirements with enteral nutrition. • earlier intervention should be considered in term infants (within 2-3 days), critically ill children (within 3-5 days), & children with preexisting malnutrition. Components of PN • PN formulations include IV sources of protein, dextrose, fat, water, electrolytes, vitamins, trace elements, etc. • Macronutrients: water, protein, dextrose, & IV fat emulsion (IVFE). • Micronutrients: vitamins, trace elements, & electrolytes. • In general, macronutrients are used for energy (dextrose & fat) & as structural substrates (protein & fat). • Micronutrients are required to support enzymatic reactions, fluid balance, & regulation of electrophysiologic processes. Osmolarity of PN Osmolarity of PN (cont’d) • The osmolarity of typical peripheral parenteral feedings is 600-900 mOsm/L. • Osmolarity of a dextrose/amino acid formulation can be approximated quickly by multiplying the % dextrose concentration by 50 & the % amino acid concentration by 100. • ~ 150 mOsm/L should be added for contribution of electrolytes, vitamins, & trace elements. • Although concurrent administration of fat emulsions (up to 60% of nonprotein calories) decreases osmolarity, buffers pH, & improves peripheral vein tolerance, it does not eliminate the risk of thrombophlebitis 2-in-1 versus 3-in-1 Macronutrient Components of PN Solutions Macronutrient Components of PN Solutions (cont’d) AAA, aromatic amino acids (includes phenylalanine and tyrosine); BCAA, branched-chain amino acids (leucine, isoleucine, and valine); EAA, essential amino acids (leucine, isoleucine, valine, phenylalanine, tryptophan, methionine, threonine, and lysine); LCT, long-chain triglycerides; PN, parenteral nutrition. Amino Acids (AAs) • Protein in PN solutions is provided as crystalline amino acids (CAAs), which are used primarily for protein synthesis. • When oxidized for energy, 1 g of protein yields 4 calories. However, including caloric contribution from protein when calculating calories provided by PN regimen is controversial. • Standard CAA solutions are designed for use in patients with "normal" organ function & nutritional requirements. • Nitrogen concentration of dietary protein is ~16% → 6.25 (100 g protein/16 g nitrogen) is commonly accepted as conversion figure for calculating nitrogen amount provided by CAA protein. Amino Acids (cont’d) • Electrolytes provided by CAA solutions must be considered when determining patient's individual requirements. • CAA are available in concentrations of 5.5-15%. • Highly concentrated products (15-20%) are attractive for use in critically ill patients who typically require fluid restriction but have large protein needs. • Modified AA solutions are designed for use in patients who have altered protein requirements, such as those with hepatic encephalopathy, renal failure, etc. • Rationale for & clinical efficacy of modified amino acids in disease-specific PN regimens is controversial. Amino Acids (cont’d) • Some conditionally EAAs, such as cysteine, carnitine, & glutamine, are not available in commercial CAA solutions in pharmacologic amounts because they are relatively unstable or poorly soluble. • → PN solutions may need to be modified by clinicians to provide desired amount of supplemental conditionally essential AAs. • Cysteine is conditionally EAA in preterm & term infants. • Carnitine use in neonatal PN regimens is generally reserved for patients receiving sole PN support for > 2 weeks. • When PN is indicated in ICU patients the amino acid solution should contain 0.2–0.4 g/kg/day of L-glutamine (e.g. 0.3–0.6 g/kg/day alanyl-glutamine dipeptide) (ESPEN). Dextrose • • • • Primary energy source in PN solutions Dextrose monohydrate, 5-70%. When oxidized, each gram provides 3.4 kcal. IV dextrose infusion rates should not exceed 12-14 mg/kg per minute in infants & 4-7 mg/kg per minute in adults. • Recommended dextrose dose for routine clinical care rarely exceeds 5 mg/kg per minute in older critically ill children (1-11 years old) & adults. • HW: what happens if dextrose infusion rate exceeds glucose oxidation rate? Intravenous Fat Emulsion (IVFE) • Concentrated source of calories & essential fatty acids. • Differ in triglyceride source (soybean oil or combination of soybean oil & safflower oil), fatty acid content, & commercially available concentrations (10, 20, & 30%). • These products also contain egg phospholipids as emulsifying agent & glycerol to make emulsion isotonic. • Caloric content: 1.1 kcal/mL for 10% emulsion, 2 kcal/mL for 20% emulsion, & 3 kcal/mL for 30% emulsion • Emulsions containing soybean oil: ~50-55% linoleic acid & 4-9% linolenic acid; IVFEs that contain safflower oil are made of ~66% linoleic acid & 4% linolenic acid. • Higher amounts of circulating phospholipids are associated with impaired triglyceride clearance in neonates & infants, 20% IVFE is the preferred product for this population. • Both IVFE types are effective for treatment or prevention of essential fatty acid deficiency (EFAD). IVFE (cont’d) • EFAD may be prevented by providing 2-5% of total calories as linoleic acid & 0.25- 0.5% as linolenic acid. • This may be achieved in most adults by giving ~100 g IVFE weekly. • Neonates & infants require minimum of 0.5-1 g/kg daily. • Risk of hypertriglyceridemia decreases with longer infusion times. • Rapid IVFE infusions are reported to contribute to ↓ oxygenation in neonates. • Adverse pulmonary effects are thought to be caused by polyunsaturated fatty acid (PUFA)-driven prostaglandin production, which results in altered vascular tone. • Rapid infusion of long-chain fatty acid formulations may have negative impact on immunocompetence by saturating reticuloendothelial system. IVFE (cont’d) • Initiation of IVFE earlier than 4-7 days of life in infants with birth weight <800 g remains controversial because of potential ↑ risk of chronic lung disease & death. • IVFE use may facilitate provision of adequate calories & minimize complications of nutrition therapy such as hyperglycemia, hepatotoxicity, or ↑ carbon dioxide production. • Patients receiving first IVFE dose should be monitored for dyspnea, chest tightness, palpitations & chills. • HA, nausea, & fever might be associated with rapid infusion rate. • IVFE use is c/i in patients with impaired ability to clear fat emulsion, such as patients with pathologic hyperlipidemia & hypertriglyceridemia associated with pancreatitis. • Patients with egg allergy should be evaluated carefully for nature & severity of reaction before deciding to initiate fatbased PN regimen. IVFE (cont’d) • 10% & 20% IVFE products may be administered either by central or peripheral route. • They may be added directly to PN solution as total nutrient admixture (TNA) or 3-in-1 system (lipids, protein, glucose, & additives), or they may be piggybacked with CAAdextrose solution. • 30% IVFE is only approved for use in preparation of TNA & is not intended for direct IV administration. • New lipid sources: medium-chain triglycerides (MCTs) are available in Europe • Advantages: MCTs are hydrolyzed & cleared > rapidly than LCTs, & they do not accumulate in liver. • MCTs do not require carnitine for entrance into mitochondria for oxidation. • However, MCTs are not source of essential fatty acids. IVFE (cont’d) • Omega-3 PUFAs (linolenic acid) are metabolized to cytokines, which may be < inflammatory & immunosuppressive than those derived from omega-6 PUFAs (linoleic acid). • Propofol is delivered in soybean oil-in-water emulsion that is essentially same as Intralipid 10%. • It is used commonly for continuous sedation of ventilated patients & should be considered a potentially significant source of calories that may require adjustment of patient's nutrition regimen. Vitamins • Adult parenteral multiple-vitamin products are available commercially. • There are two commercially available parenteral vitamin products for use in pediatric patients: MVI-Pediatric & Infuvite Pediatric for infants weighing <1 kg to children up to 11 years old. • There are no commercially available IV multivitamin products designed to specifically meet unique requirements of premature infants (higher vitamin A & lower doses of vitamins B1, B2, B6, & B12 compared to recommendations for term infants & older children). Vitamins (cont’d) • Parenteral multiple-vitamin formulations for adults contain 13 essential vitamins. • Supplemental vitamin K may be given IM or SC or added to PN solution if needed. • Current recommendations suggest supplemental vitamin K is unnecessary when vitamin K-containing multiplevitamin product is used. • Parenteral multiple-vitamin formulation containing no vitamin K is commercially available for use in patients receiving home parenteral nutrition & warfarin anticoagulation. • Individual & combination products are available to provide additional or tailored supplementation (to prevent vitamin toxicities or deficiencies caused by altered metabolism or drug therapy). Trace Elements • IV trace elements are available as single-mineral solutions & as multiple-mineral combinations ±electrolytes. • Most products provide daily requirements for zinc, copper, chromium, & manganese, whereas some also include iodide, molybdenum, or selenium. • Higher doses of supplemental zinc likely are necessary in patients with high-output ostomies or diarrhea because GIT is predominant excretion route for zinc. • Manganese & copper are excreted through biliary tract → should be restricted or withheld from PN solutions in patients with cholestatic liver disease • Chromium, molybdenum, & selenium are excreted renally → should be restricted or withheld in patients with renal failure Electrolytes • Patients who have "normal" organ function & relatively normal serum concentrations of any electrolyte should receive normal maintenance multiple-electrolyte doses when PN is initiated & daily thereafter. • Concentrated multiple-electrolyte solutions designed for addition to PN solutions generally contain only sodium, potassium, calcium, & magnesium. • Phosphorus must be added as separate additive. Designing PN Regimen • Several factors are important: venous access, fluid status, & macronutrient & micronutrient requirements. • Patient's venous access & fluid status determines how concentrated PN solution may be compounded. • PN solutions may be administered by central or peripheral venous access depending on clinical condition. • PN solutions may be provided as a 2-in-1 formulation that contains dextrose, CAA, & other necessary micronutrients, or as TNA that contains dextrose, CAA, & IVFE, as well as other necessary micronutrients. • Advantages of TNA solutions: ↓ infusion pumps, tubing, etc.), ↓ time for compounding & administration, ↓ in manipulations of infusion line (↓ risk of catheter contamination), ease of delivery & storage for patients receiving home PN. • Potential disadvantages: ↑risk of infections & stability & compatibility concerns → < desirable in specific patient populations such as neonates & infants. Route of PN & infusion type depend on patient's clinical status & expected length of therapy. Routes of PN Administration Peripheral Route (PPN) • Is option for mild to moderately stressed patients in whom central access is unavailable or undesirable & function of their GIT is expected to return within 10 -14 days. • Lower concentrations of AAs (3-5% final concentration), dextrose (5-10% final concentration), & micronutrients & larger volumes compared to central parenteral nutrition (CPN) are necessary → patient should not be fluid restricted or require large nutrient amounts. • Although dilute with nutrients, osmolarity of these formulations is 600-900 mOsm/L (hypertonic) → are irritating to peripheral veins & can cause thrombophlebitis → necessitates frequent site rotations (at least every 48–72 hours), which may quickly exhaust venous access sites. • Advantages: lower risk of infectious, metabolic, & technical complications. Routes of PN Administration (cont’d) Peripheral Route (PPN) • Factors complicating PPN: multiple courses of chemotherapy, malnourished patients, premature infants, elderly patients, & others after multiple venous access. • Thrombophlebitis is common complication. • To minimize phlebitis: addition of IVFE to regimen as possible venous lumen protectant, subtherapeutic heparin doses (0.5 to 1 unit/mL) to prevent thrombus formation, &/or small doses of hydrocortisone (5 mg/L) to minimize access site inflammation. • Midline catheter use may offer some advantage with reducing risk of thrombophlebitis (they are longer & infuse into larger venous vessels which may dilute PN solution to more tolerable osmolarity. Central Route • CPN is the preferred choice for PN delivery & is used for patients who - require PN for periods >7–14 days during hospitalization or indefinitely at home. - have large nutrient requirements, - poor peripheral venous access, - fluctuating fluid requirements (metabolically stressed patients with extensive surgery, trauma, sepsis, multipleorgan failure, or malignancy). • CPN solutions are highly concentrated hypertonic solutions that must be administered through large central vein. • Disadvantages: risks associated with catheter insertion, routine catheter use & care of access site. Central Route (cont’d) • Central venous catheters for short-term use in adults are commonly inserted percutaneously into subclavian (or peripheral like basilic, cephalic, or brachial) vein & advanced so that the tip is at superior vena cava. • If this approach is not possible, internal jugular vein can be used. • In neonates: via catheter placed in umbilical vein. • When therapy is expected to last >4 weeks, catheter usually is tunneled subcutaneously before entering central vessel, secured initially with retaining sutures, & anchored in place with cuff. • Parenteral nutrient formulations designed for administration through central veins can contain relatively high concentrations of dextrose (20%–35%), amino acids (5%–10%), and lipids providing a caloric density of >1 kcal/mL in a solution with an osmolarity of >2,000 mOsm/L Adult PN Solutions 2 methods for ordering adult PN: 1. “Standard formula approach" - base formulations with fixed nonprotein calorie–to-nitrogen ratio. • usually includes different formulas designed for mild to moderately stressed patients, renal failure patients, fluidrestricted patients, & liver failure patients. • Because nonprotein calorie-to-nitrogen ratio is fixed, amount of nutrient delivered depends solely on infusion rate. 2. “Individualized formula approach" permits compounding of patient-specific solutions. • Compounding of PN solution is limited only by concentrations of stock solutions & stability of additives. • Nutrient amount delivered depends on daily volume of PN solution infused & nutrient concentrations in PN solution. Calculation of Adult PN Regimen • Patient case: Patient’s daily nutritional requirements are 100 g protein & 2,000 total kcal. • Patient has central venous access & reports no history of hyperlipidemia or egg allergy. • Patient is not fluid restricted. • PN solution will be compounded as individualized regimen using single-bag, 24- hr infusion of 2-in-1 solution with IVFE piggybacked into PN infusion line. • Determine total PN volume & administration rate by calculating macronutrient stock solution volumes required to provide desired daily nutrients. • Stock solutions used to compound this regimen are 10% crystalline amino acids (CAA), 70% dextrose, & 20% IVFE. Calculation of Adult PN Regimen (cont’d) 1. Determine daily IVFE calories & volume • 2,000 kcal/day × 30%–40% of total calories as fat = 600– 800 kcal/day • Choose IVFE 20% 250 mL/day × 2 kcal/mL = 500 kcal/day 2. Determine 70% dextrose stock solution volume • Determine dextrose calories • Dextrose calories = TOTAL − IVFE − Protein • 2,000 kcal − 500 kcal IVFE − (4 kcal/g × 100 g CAA) = 1,100 kcal • Calculate required dextrose (grams) • 1,100 kcal ÷ 3.4 kcal/g dextrose = 324 g dextrose • Determine 70% dextrose volume • 70 g/100 mL = 324 g/X mL 70% dextrose; X = 463 mL 70% dextrose Calculation of Adult PN Regimen (cont’d) 3. Calculate 10% CAA stock solution volume • 10 g/100 mL = 100 g/X mL 10% CAA; X = 1,000 mL 10% CAA 4. Determine 2-in-1 PN volume & administration rate • Calculate CAA/dextrose volume • 463 mL 70% dextrose + 1,000 mL 10% CAA = 1,463 mL CAA–dextrose • Add 100–200 mL for additives • Total 2-in-1 volume = ~1,600–1,700 mL/day • Calculate administration rate • 1,600–1,700 mL/day ÷ 24 hours = 67–71 mL/hour; round to 65–70 mL/hour Calculation of Adult PN Regimen (cont’d) 5. Choose final 2-in-1 PN regimen & determine provided • • • • • • • • nutrient amounts: Final 2-in-1 regimen 100 g CAA/324 gm dextrose in 1,680 mL/day to infuse at 70 mL/hour + 20% IVFE 250 mL to infuse at 2 mL/hour Calculate macronutrient calories 20% IVFE calories: 250 mL × 2 kcal/mL = 500 kcal Dextrose calories: 324 g × 3.4 kcal/g = 1,102 kcal Protein calories: 100 g × 4 kcal/g = 400 kcal Total kcal: 2,002 kcal Nonprotein kcal: 1,602 kcal Simplifying calculation of PN regimen after patient's nutritional requirements have been decided • Adult patients receiving only PN therapy may need larger volumes of fluid to provide maintenance requirements & replace extrarenal losses. • Patients may receive adequate fluid from additional IV maintenance solution (e.g., 0.45% NaCl in 5% dextrose) &/or piggybacked medications. • Methods for estimated fluid needs for basic maintenance: 1. The simplest method uses 30-35 mL/kg/day as the basis. 2. Another method is to provide 1,500 mL for the first 20 kg body weight plus additional 20 mL/kg for actual weight beyond the initial 20 kg. • Additional fluid must be provided for increased losses such as vomiting, nasogastric (NG) tube output, diarrhea, or large open wounds. Pediatric PN Solutions • Are typically ordered using individualized approach based on patient's weight. • Current safe practice guidelines suggest that pediatric PN label identify components as "amount per day" with secondary expression of components as "amount per kilogram per day.“ • Because infants & children generally receive daily maintenance fluid from PN regimen, supplemental IV solutions are rarely needed. • TNA system is not recommended for use when compounding neonatal PN because of IVFE instability with higher calcium & phosphorus concentrations. Administration Techniques • PN solutions should be administered with infusion pump. • IV administration line for CAA-dextrose solutions should include 0.22-micron inline filter to remove particulate matter, air, & any microorganisms. • IVFEs administered separately from CAA-dextrose solution must be piggybacked into PN line at site beyond inline filter because average size of IVFE particles is ~0.5 microns. • FDA recommends for IVFE use of 1.2-micron filter, which may be effective in preventing catheter occlusion caused by precipitates or lipid aggregates • This filter size is also reported to remove Candida albicans. Initiating & Advancing PN Infusion Adult PN • Patient's nutrition status, current clinical status, history of glucose tolerance, & dextrose concentration in formula will dictate infusion rate. • Stable patients with normal organ function & stable baseline serum glucose concentrations: abruptly initiating or discontinuing PN therapy. • Patients receiving intermittent SC regular insulin, patients with severe renal or hepatic disease, other disease states that may ↑ risk of hypoglycemia (severe diabetes or pancreatic malignancy & patients who are receiving betablockers): begin PN infusion &↑ rate gradually over 12-24 hours to desired rate →↓ in stepwise fashion, e.g., by 50% for 1 hour prior to discontinuation Continuous versus Cyclic Infusions • Continuous infusions are attractive for use in patients with unstable fluid balance or glucose control. • Intermittent or cyclic infusion of PN, usually for 12-18 hours each day, is useful in hospitalized patients with limited venous access in whom administration of multiple other medications requires interruption of PN infusion. • Cyclic PN also may prevent or treat hepatotoxicities associated with continuous PN therapy. • In addition, this delivery mode allows patients receiving PN at home ability to resume relatively normal lifestyle. • Cyclic PN is not optimal for all patients & should be used with caution in those with severe glucose intolerance or diabetes, or unstable fluid balance. Evaluation of Therapeutic Outcomes • Serum concentrations of electrolytes, hematologic indices, & biochemical markers for renal function, liver function, & nutrition status should be measured prior to PN initiation & periodically thereafter depending on patient's age, nutrition status, & clinical condition. • Frequency of blood tests in neonates & infants tends to be more conservative due to smaller circulating blood volumes &, in some cases, lack of central vascular access. • Other important clinical measurements include vital signs, weight, total fluid intake & losses, & nutritional intakes. • Weekly height/length & head circumference measurements are helpful for monitoring nutritional changes in neonates. Monitoring strategy for patients receiving PN. • Baseline (prior to initiation of TPN) ↓ Weight Vital signs • Temperature • Pulse • Respirations Current nutritional intake CBC Serum electrolytes • Sodium • Potassium • Chloride • Bicarbonate • Magnesium • Phosphorous • Calcium Serum glucose Serum albumin Markers for organ function • LFT • AST • ALT • Total bilirubin • PT or INR RFT • Blood urea nitrogen • Creatinine Fluid balance - Input • Oral • Nasointestinal • Intravenous - Output • Urine • Gastrointestinal • Other losses Special pediatric considerations: height/length, head circumference Monitoring strategy for patients receiving parenteral nutrition (PN) (cont’d) Daily ↓ • Vital signs • Current nutritional intake • Fluid balance - Input - Output • Capillary blood glucose - As necessary; unstable patients may require measurements every 1–2 h ↓ Serum electrolytes • Sodium • Potassium • Chloride • Bicarbonate • Calcium Serum glucose ↓ ↓ Daily for first 3–4 days after initiation of TPN • Add magnesium & phosphorous if patient at risk for refeeding syndrome or if baseline values are abnormal • Serum electrolytes may be measured less frequently in stable patients • Critically ill patients may require more frequent monitoring • Special pediatric considerations: • Total bilirubin—daily in newborns until normal Monitoring strategy for patients receiving parenteral nutrition (PN) (cont’d) • Weekly ↓ Nitrogen balance Prealbumin or transferrin Serum triglyceride Liver function tests • AlkP • AST • ALT • Total bilirubin • PT or INR (as necessary) ↓ • Liver function tests may be drawn less frequently in stable patients (every 2–4 weeks) ↓ Special pediatric considerations: • Height/length • Head circumference Compounding, Storage, & Infection Control • Compounded sterile preparations are defined by risk level (high, medium, low) based on probability of microbial, chemical, or physical contamination. • PN solutions are classified as medium-risk compounded sterile preparation. • In general, PN solutions should be prepared using aseptic technique under properly maintained laminar flow hood. • Supervision by pharmacist experienced in compounding IV solutions & knowledgeable about stability, compatibility, & storage of PN solutions is also necessary. Compounding, Storage, & Infection Control (cont’d) • Quality assurance procedures should be developed to maintain safe & accurate admixture preparation. • PN base solutions may be prepared by using gravity-driven transfer of CAA stock solutions to partially filled bags of concentrated dextrose stock solutions. • There are commercially prepared CAA-dextrose products separated within single bag & then mixed prior to use. • Advances in compounding technology have facilitated use of automated compounders for preparing PN solutions perform calculations necessary to determine volumes of nutrient stock solutions → communicates determined calculations directly to transfer pump device that delivers fluid from source container to final container by either volumetric or gravimetric fluid pumping system. Compounding, Storage, & Infection Control (cont’d) • In spite of acidic pH & hypertonicity, growth of Pseudomonas aeruginosa, Escherichia coli, & C. albicans has been reported in CAA-dextrose solutions. • TNA solutions appear to support growth of bacteria less than IVFEs, but more than CAA-dextrose solutions. • CDC recommends that IVFE infusions not exceed 12 hours, unless volume considerations require more time, in which case IVFE infusions should be completed within 24 hours. • But FDA-approved guidelines for handling procedures for IVFE-containing anesthetic agent propofol restrict infusion time to 12 hours after bottle has been spiked & require infusion tubing change every 12 hours. Compounding, Storage, & Infection Control (cont’d) • CDC guidelines recommend use of administration sets for 2-in-1 PN for up to 72 hours, but those used for TNA solutions & IVFE should be changed every 24 hours. • Frequently neonates require considerably smaller volumes (e.g., 2 ml/day) of IVFE than are commercially available. • Some institutions aseptically transfer IVFE into plastic syringes for syringe pump infusion →↑ risk of bacteremia. • FDA-approved guidelines for propofol handling procedures restrict infusion times for doses provided in syringes to 6 hours. • Many institutions allow infusion times between 12 & 24 hours. Stability & Compatibility • CAA-dextrose solutions generally are stable for 1-2 months if refrigerated at 4°C & protected from light. • TNA formulations are complex mixtures that are inherently unstable. • Several factors affect stability of TNA solutions, including pH, electrolyte charges, temperature, & time after compounding. • US Pharmacopeia 797 standards recommend storage times of not > 30 hours at controlled room temperature (15°C to 30°C) & not more than 9 days at refrigerated temperatures (2°C to 4°C) for all medium-risk compounded sterile preparations, including PN solutions. Stability & Compatibility (cont’d) • Because of differences in pH among various CAA products & differences in phospholipid content among IVFE products, specific manufacturers should be consulted for compatibility & stability information prior to routine mixing of components. • One approach to compounding TNA formulations manually is to first combine CAA, dextrose, & sterile water (if necessary). • Add electrolytes, vitamins, & trace elements & then visually inspect solution for precipitate. • Finally, add IVFE & visually inspect solution to ensure uniform emulsion exists. • But: this specific order & time sequence may not be possible with use of automated compounders. Stability & Compatibility (cont’d) • Precipitation of calcium & phosphorus is common interaction that is potentially life-threatening. • Factors that enhance risk of precipitate formation include high concentrations of calcium & phosphorus salts, use of chloride salt of calcium, decreased amino acid & dextrose concentrations, increased solution temperature, increased solution pH, use of improper sequence when mixing calcium & phosphorus salts, & presence of other additives including IVFEs. • Electrolyte stability in TNA solutions is difficult to assess because of poor visualization of precipitate should one occur. • PN solutions for neonates & infants tend to have larger calcium & phosphorus amounts, as well as other divalent cations, that limit use of TNA formulations. • Use of 2-in-1 formulation with separate administration of IVFEs is recommended for neonates & infants. Stability & Compatibility (cont’d) • Addition of bicarbonate to acidic PN solutions → carbon dioxide gas & insoluble calcium & magnesium carbonates → sodium bicarbonate use in PN solutions is not recommended (use bicarbonate precursor such as acetate). • Because of variable stabilities of individual vitamins, IV vitamin solutions should be added to PN solution near to time of administration, & should not be in PN solution longer than 24 hours. • Peroxide formation in dextrose-amino acid solutions depends on concentration of IV multivitamins, CAA, & dextrose, & presence of IVFEs. • Peroxides have negative effects on organ & immune function: e.g., neonatal hypoxic–ischemic encephalopathy, intraventricular hemorrhage, chronic lung disease, retinopathy of prematurity & necrotizing enterocolitis. • Protecting PN & IVFE solutions from light is recommended to minimize peroxide formation. Stability & Compatibility (cont’d) • Compatibility of IV medications & other IV solutions is an important concern in delivering safe & effective drug and nutritional therapy. • IV medications are infused most often as separate admixture piggybacked in PN line. • For adding medications directly to PN solution, specific criteria should be considered: dosage regimen should be stable for each 24-hour period & should have pharmacokinetic properties appropriate for continuous infusion. • There should be documented chemical & physical compatibility of medication with PN mixture. • PN regimen should be infused continuously over 24 hours. HW: • Advantages & disadvantages of PN admixtures with drugs? • Examples of drugs frequently used as such admixtures Complications of PN 1. Mechanical or Technical Complications • infusion pump failure, • problems with administration sets or tubing, • problems with catheter - potentially life-threatening: - pneumothorax, - catheter misdirection or migration into wrong vein or improperly positioned within cardiac chambers, - arterial puncture, - bleeding, - hematoma formation, - venous thrombosis, - air embolism, - breakage of catheter Complications of PN (cont’d) 2. Infectious Complications • Can be major hazard in patients receiving CPN. • Predisposing factors: compromised immunity, concomitant infection, frequent use of broad-spectrum antibiotics, malnutrition • Sources of infection: improper preparation of solutions, catheter-related bloodstream infection (defined as presence of bacterial or fungal growth from catheter tip & peripheral blood cultures). • Catheter infection or colonized catheter is defined as microbial growth from catheter tip or from blood culture drawn from catheter with no growth of the same organism in peripheral blood culture. • Colonization may occur after multiple manipulations of line used for PN administration (when used to administer other medications), failure of in-line bacterial filters, poor placement technique, & poor care of insertion site. Complications of PN (cont’d) 3. Metabolic & Nutritional Complications • PN-associated hepatic dysfunction • Risk factors in children: degree of prematurity, sepsis, hypoxia, lack of enteral nutrition, small bowel bacterial overgrowth, GI conditions requiring surgical intervention, duration of PN therapy, long-term administration of excessive calories. • PN-associated hepatic dysfunction in infants is characterized clinically by serum direct bilirubin concentration > 2 mg/dL. • Taurine deficiency has been proposed as etiology of cholestasis in preterm infants & neonates (essential amino acid not present in standard CAA solutions but important in neonatal & infant bile metabolism). • Risk factors in adults: preexisting liver diseases, sepsis, preexisting malnutrition, extensive bowel resection, duration of PN therapy, lack of enteral intake, choline deficiency, long-term administration of excessive calories Metabolic Abnormalities Associated with PN Macronutrients ALT, alanine aminotransferase (SGPT); AST, aspartate aminotransferase (SGOT); Alk Phos, alkaline phosphatase; Bili, bilirubin; EFAD, essential fatty acid deficiency; EN, enteral nutrition; IVFE, intravenous fat emulsion; PN, parenteral nutrition. Complications of PN (cont’d) Metabolic & Nutritional Complications (cont’d) • Hypertriglyceridemia (TG 400-500 mg/dL in adults & 150-200 mg/dL in preterm infants, neonates, & older pediatric patients) may occur in patients receiving IVFEbased PN. • Risk factors: preexisting liver or pancreatic dysfunction, sepsis, multiple-organ failure, degree of prematurity, IVFE infusion rate, & dose. • IVFE-associated hypertriglyceridemia generally is thought to be caused by defective lipid clearance. • Reducing IVFE infusion rate or dose or withholding IVFE therapy should be considered when patients present with hypertriglyceridemia or lipemic serum. • Use of low-dose heparin to stimulate lipoprotein lipase activity has been suggested. Complications of PN (cont’d) Metabolic & Nutritional Complications (cont’d) • Severe & rapid ↓ in serum phosphate, potassium & magnesium concentrations, fluid retention are common features of refeeding syndrome. • Individuals at risk: severely malnourished with significant weight loss who receive aggressive nutritional supplementation, unfed for 7-10 days with evidence of stress or nutritional depletion, chronic diseases causing undernutrition (cancer, cardiac cachexia, COPD or cirrhosis), & those with previous morbid obesity & massive weight loss. • Recommendations for initiating PN in adults at risk for refeeding syndrome: providing 25-50% of calculated nonprotein caloric requirements initially. Complications of PN (cont’d) Metabolic & Nutritional Complications (cont’d) • Dextrose dose should be initiated at ~100-200 g/day. • Calories should be advanced over 3-4 days to desired goal. • Pediatric PN regimens are usually advanced over several days as general practice. • Additional recommendations for minimizing risk of refeeding syndrome in pediatric patients includes provision of additional phosphorus & potassium above standard nutrient requirements at the time PN is initiated HOMEWORK: • Hyperglycemia & its management/prevention (including insulin doses & glycemic targets in critically ill patients) Complications of PN (cont’d) Metabolic & Nutritional Complications (cont’d) • Lactic acidosis & other life-threatening complications associated with severe thiamine deficiency in patients who received PN solutions without multivitamin supplementation → at least maintenance doses of vitamins, trace elements & EAAs should be provided to all patients with normal age-related organ function receiving PN. • Patients receiving PN regimens without IVFEs for extended periods (weeks-months) are at risk for development of EFAD: hair loss, desquamative dermatitis, thrombocytopenia, malabsorption & diarrhea resulting from changes in intestinal mucosa. • Triene-to-tetraene ratio > 0.4 is biochemical evidence for EFAD. • May occur within 72 hours in premature infants. Complications of PN (cont’d) Metabolic & Nutritional Complications (cont’d) • Metabolic bone disease is reported in adults & children receiving long-term home PN. • In adults is characterized by osteomalacia with or without osteoporosis • Diagnosis may not be made in premature infants until after development of bone fractures or overt rickets. • Etiology is likely multifactorial. • Treatment: calcium & vitamin D supplementation & exercise. • Others have recommended removal of vitamin D from PN in patients with low serum PTH & 1,25-hydroxyvitamin D concentrations. • Nutrient-induced toxicities, most commonly as result of accumulation of fat-soluble vitamins or trace elements Complications of PN (cont’d) Metabolic & Nutritional Complications (cont’d) • Aluminum accumulation may occur during long-term PN therapy, especially in patients with renal insufficiency, & is associated with abnormal neurologic & hematologic function & metabolic bone disease in adults & premature infants. • FDA: parenteral doses of 4-5 mcg/kg per day are associated with CNS & bone toxicity in patients with impaired renal function, including premature • In 2004 FDA: restriction of aluminum content in largevolume PN stock solutions (CAA, dextrose, sterile water for injection, IVFE) to maximum of 25 mcg/L & requirement for manufacturers to indicate maximum aluminum concentration at expiration for both large & small volume parenteral products used for PN.