Elements
advertisement

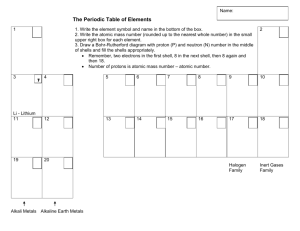
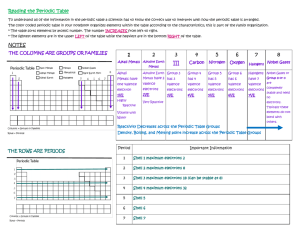
START LESSON TEACHER'S NOTES Teacher's Notes Subject: Chemistry Topic: The periodic table Grades: 7-9 Prior Knowledge: The atomic structure of elements. Lesson Objectives By the end of this lesson students should understand: ·electron shells ·valence shells ·how atomic numbers are derived by the number of protons in the atomic structure ·how the periodic table is organized by atomic number, number of electron shells (rows), and number of protons in the valence shell (columns) ·how the periodic table is organized into different families of properties Lesson Notes Dmitri Mendeleev published the first periodic table in 1869. This lesson is a brief introduction to the periodic table. Major lesson points: ·The atomic number is based on the number of protons in an element's atomic structure. ·In a natural state, an element has an identical number of neutrons. Neutrons are not discussed here, however. protons, electrons, and ·Elements are often arranged in columns according to the number of electrons in the valence or outer shell of an atom. ·Transition metals, lanthanoids (or lanthanides) and actinoids (or actinides) look at the electrons orbiting beneath the outer to understand their atomic structure. shell to subshells The lesson concludes with an assessment of the students learning of the lesson concepts and objectives. The periodic table is designed to classify the elements. Let’s start with Hydrogen and Helium. Touch the sun, then drag to the terms to the correct line, then drag hydrogen to box 1 and helium to box 2. Sorting four more elements. Pull the tabs, read the descriptions, then move each atom to its correct place. Hydrogen has one proton, therefore, its atomic H number is 1. He Lithium has 3 protons. 2 electrons reside in its inner Li with 1 on the valence shell. Move lithium to it's shell correct place. Ne Sodium has 11 protons. 2 electrons are in the inner shell, 8 in the 2 shell, Na just 1 in the valence shell. Move sodium to its correct place. and nd Ar Placing more elements on the periodic table. Columns 1-2 and 13-18. Touch each element to enlarge. Study their valence shells. Placing more elements on the periodic table. Columns 1-2 and 13-18. Touch the elements on the right to reveal their atomic structure. Pull and read each tab. Si Selenium has 34 protons. 2 electrons are in the inner shell, 8 in the 2nd shell, Se 18 in the 3rd shell, and 6 in the valence shell. Move selenium to its correct place on the chart. Cl Gallium has 31 protons. 2 electrons are in the inner shell, Ga 8 in the 2nd shell, 18 in the 3rd shell, and 3 in the valence shell. Move gallium to its correct place on the chart. Uup Calcium has 20 protons. 2 electrons are in the inner shell, 8Cain the 2nd shell, 8 in the 3rd shell and 2 in the valence shell. Move calcium to its correct place on the chart. Placing more elements on the periodic table. Columns 1-2 and 13-18. Placing more elements on the periodic table. Columns 3-12. Study each atom and their valence shells. Move them to the correct box on the chart. The elements in these sections have either 0, 1, or 2 electrons in their valence shells. This makes it difficult to find the correct column for each element. In an element's standard form, there is one electron for each proton. Add the column of electrons to determine the number of protons and atomic number for each element. Elements have different properties influenced by their molecular structure. Touch each colored area to learn about its unique properties. Click here to classify columns 13-16 in another way. Halogens Oyxgen family Actinoids Nitrogen family Lanthanoids Carbon family Transitional Metals Boron family Alkali metals Within column 3, rows 6 and 7 have many elements that are very similar in properties. In order to conserve space, two additional rows have been created at the bottom, representing elements 57-71 (lanthanoids) and 89-103 (actinoids). Noble Gases Alkaline Earth metals Version #1-(for Boron, Carbon, Nitrogen, Oxygen families) Alkali metals Alkali metals: These elements have 1 electron in the valence shell. They react easily with other elements. These are not found in elemental form in nature. They are lighter and softer than alkalines. Alkaline Earth Metals Alkaline Earth Metals: These elements have 2 electrons in the valence shell. They react easily with other elements. They form ionic salts with halogens. They are heavier and harder than alkali metals. Transitional Metals Transition Metals have properties that reflect their unique electron structure. Note that the valence shell holds 0, 1, or 2 electrons but the inner shell has a differing count of electrons. Transition Metals are very hard. They have high melting and boiling points. They conduct electricity. Boron Family Boron Family: Elements in this family contain 3 electrons in the valence shell. These elements form stable bonds with other elements. Carbon Family Carbon Family: Elements in this family contain 4 electrons in the valence shell. These elements form stable bonds with other elements. Nitrogen Family Nitrogen Family (also known as pnictogen): Elements in this family have 5 electrons in the valence shell. Oyxgen Family Oxygen Family (also known as chalcogen): Elements in this family have 6 electrons in the valence shell. Halogens Halogens: These elements have 7 electrons in their valence shell and are highly reactive. They can be harmful or lethal to biological organisms and some are used as disinfectants. Noble Gases Noble gases have a full valence shell and are very stable and nonreactive with other elements. Electric currents cause these gases to light up. Noble gases are used as refrigerants because of their low boiling and melting points. Lanthanoids Lanthanoids: The first additional row, elements 57-71, are named after the first element in this group, lanthanum. Because lanthanoids deflect ultraviolet and infrared radiation, they are used in sunglasses. They are also used in catalytic converters and as catalysts to refine petroleum. Actinoids Actinoids: The second additional row, elements 89-103, are named after the first element in this group, actinium. All actinoids are radioactive. Since most actinoids are difficult to find naturally, scientists synthesize them in laboratories. Supernovae, nuclear reactors and nuclear explosions also create actinoids, which then decay. Elements have different properties influenced by their molecular structure. Touch each colored area to learn about its unique properties. Click here to classify columns 13-16 in another way. Lanthanoids Actinoids Noble Gases Transitional Metals Halogens Alkali metals Alkaline Earth metals Version #2-(for Nonmetal, Metalloid, Poor) Alkali metals Alkali metals: These elements have 1 electron in the valence shell. They react easily with other elements. These are not found in elemental form in nature. They are lighter and softer than alkalines. Alkaline Earth metals Alkaline Earth Metals: These elements have 2 electrons in the valence shell. They react easily with other elements. They form ionic salts with halogens. They are heavier and harder than alkali metals. Transitional Metals Transition Metals: Have properties that reflect their unique electron structure. Note that the valence shell holds 0, 1, or 2 electrons but the inner shell has a differing count of electrons. Transition metals are very hard. They have high melting and boiling points. They conduct electricity. Poor Poor (or Post Transition): These metals are softer with lower melting and boiling points than transition metals. Metaloids Metalloids: These elements can react as an acid or base. They often behave as semiconductors. Nonmetals Non-metals: These elements conduct heat and electricity poorly, they have significantly lower melting and boiling points, and lower densities. Hydrogen is classified as a non-metal. Halogens Halogens: Elements have 7 electrons in their valence shell and are highly reactive. They can be harmful or lethal to biological organisms and some are used as disinfectants. Noble Gases Noble gases have a full valence shell and are very stable and nonreactive with other elements. Electric currents cause these gases to light up. Noble gases have low boiling and melting points and are used as refrigerants. Lanthanoids Lanthanoids: The first additional row, elements 57-71, named after the first element in this group, lanthanum. Because lanthanoids deflect ultraviolet and infrared radiation they are used in sunglasses. They are also used in catalytic converters and as catalysts to refine petroleum. Actinoids Actinoids: The second additional row, elements 89-103, named after the first element in this group, actinium. All actinoids are radioactive. Since most actinoids are difficult to find naturally, scientists synthesize them in laboratories. Supernovae, nuclear reactors and nuclear explosions also create actinoids, which then decay. 1 The atomic number is ___________. A B C D the number of neutrons in an atom the weight of an atom the total number of protons, neutrons, and electrons in an atom the number of protons in an atom In its standard form, an element will have twice as many electrons as protons. 2 A B True False 3 Noble gases are nonreactive. A B True False 4 All elements are found naturally on Earth. A B True False Elements are not found outside of Earth's atmosphere. 5 A B True False 6 What is a valence shell? A B C D The outer shell of an atom. The proton shell of an atom. An inner shell of an atom. A middle shell of an atom. Place the have following words in a column Elements different properties influenced beneath the two images: by their molecular structure. Atomic #1 Nonreactive Hydrogen Helium Reactive Atomic #2 Answer have different properties influenced Elements by their molecular structure. Atomic #1 Atomic #2 Hydrogen Helium Reactive Nonreactive The periodic table primarily sorts elements according to which criteria: 7 A gases, liquids, solids. B valence shell electrons. C natural and synthetic. D metals and nonmetals. Which of the following represents this atom on the periodic table? 8 A Gold B Phosphorus C Iron D Copper