Gas Laws
advertisement

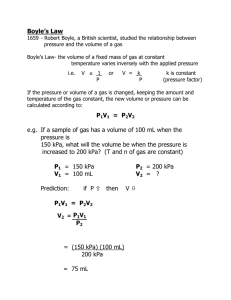
Kinetic Molecular Theory (KMT) Gases consist of very small particles (atoms or molecules) which are separated by large distances. Most of the volume occupied by a gas is empty space. Gas molecules move randomly at very high speeds in all directions. Pressure results from molecules colliding with the walls of the container. 5-1 Kinetic Molecular Theory (KMT) There are no intermolecular forces between the gas molecules except when they collide. All collisions between molecules are elastic collisions. The internal energy of the system remains the same. The KE of molecules is dependent upon the absolute temperature. 5-2 STP Standard temperature and pressure, T = 273.15K ≈ 273K P = 1 atm = 760 mm Hg = 76 cm Hg 1.0 mol of any gas = 22.4 L = 22.4 dm3 5-3 The Gaseous Phase In the gaseous state, the molecules have sufficient energy to overcome the intermolecular forces that attract them to each other. Each molecule acts independent of the others. Gases have low densities and completely fill the volume that is available to them. 5-4 Units Used In Measuring Gases Volume mL, L, cm3, dm3, m3 Temperature Must use K in gas laws. Pressure atm, torr, kPa, Pa Moles Amounts measured in moles. 5-5 What Makes a Gas an Ideal Gas? Any ideal gas assumes the: volume that the gas molecules themselves occupy is neglected. gas molecules do not attract each other. Ideal gases are approximated when the intermolecular attractions are very small. 5-6 Low pressures and high temperatures are the conditions by which a gas approaches the ideal. Low pressures enable gas molecules to spread very far apart. High temperatures represent a large amount of kinetic energy which overcomes attractive forces. Small gas molecules such as hydrogen with only dispersion forces best approximate the ideal. 5-7 Deviations from the ideal gas law occur most at low temperatures and high pressures. The ideal gas law (IGL) is given by PV = nRT L•atm , where the gas constant R = 0.0821 mol•K P is the pressure in atm, V is the volume in L, n is the number of moles, and T is the Kelvin or absolute temperature. 5-8 Ideal Gas Law Problems What is the pressure exerted by 31.0 moles of carbon dioxide that is contained in a 110. liter tank at 28°C? n = 31.0 moles V = 110. L R = 0.0821 T = 28°C = 301 K L•atm mol•K 5-9 PV = nRT P = L•atm 31.0 mol × 0.0821 mol•K × 301 K 110. L P = 6.96 atm 5 - 10 A gas with a mass of 3.47 g occupies 282 mL at 23.0°C and 743 mm Hg. Determine the molecular mass of the gas. m = 3.47 g V = 282 mL T = 295 K L•atm P = 743 mm Hg R = 0.0821 mol•K m PV = nRT n= MM mRT MM = PV 5 - 11 MM = L•atm 3.47 g × 0.0821 mol•K × 295 K 743 mm × 1 atm × 282 mL × 1 L 760 mm 103 mL MM = 305 g•mol-1 5 - 12 Charles’s Law Charles’s law says that at constant pressure, the volume of a gas is directly proportional to the absolute temperature (K). Remember the gas laws require temperatures to be expressed in Kelvin to avoid dividing by zero. 5 - 13 Two quantities, V and T, are directly proportional if by whatever factor T changes, V changes by the same factor. A graph of V vs T yields a straight line through the origin. The following graph looks to be an exception but it is not because the temperature is expressed in °C. 5 - 14 The graph shows that when the line is extrapolated to -273.15°C, you expect the volume to be zero. At 0 K, a gas is predicted to have a zero volume but all gases either liquefy or solidify before reaching that temperature. 5 - 15 . -273.15 °C = 0 K (absolute zero) 5 - 16 Deriving Charles’s Law Charles’s law can be derived from the ideal gas law. Assume the same gas has existed at two different temperatures while the pressure and the number of moles of gas remained constant. 5 - 17 Applying the IGL at the two different temperatures yields: P1V1 = nRT1 P2V2 = nRT2 Dividing one equation by the other yields: P1V1 P2V2 V1 T1 = nRT1 nRT2 V2 = T2 => P1V1 P2V2 = nRT1 nRT2 (Charles’s Law) 5 - 18 Charles’s Law Problem A sample of oxygen has a volume of 350 mL at 60.0°C. What will be the volume of oxygen at standard temperature if the pressure remains constant? V1 = 350 mL T1 = 60.0°C = 333 K P1 = P2 V2 = ? T2 = 0.0°C = 273 K P2 = P1 5 - 19 . V1 T1 V2 = T2 V1 V2 = × T2 T1 V2 = 350 mL × 273 K = 290 mL O2 333 K 5 - 20 Boyle’s Law Boyle’s law says that at a constant temperature for a fixed quantity of gas, the volume of the gas is inversely proportional to the pressure. Two quantities, P and V, are inversely proportional because by increasing either variable by a factor causes a decrease in the other variable that was the inverse of the first factor. 5 - 21 A graph of V vs P yields a hyperbola. When the pressure is doubled (2 ×), the volume is halved (1/2). 5 - 22 A graph of V vs 1/P yields a straight line. 5 - 23 Deriving Boyle’s Law Boyle’s law can be derived from the ideal gas law. Assume the same number of moles of gas has remained at the same temperature while the pressure and volume have varied. 5 - 24 Applying the IGL at the two different pressure yields: P1V1 = nRT1 P2V2 = nRT2 Dividing one equation by the other yields: P1V1 P2V2 = nRT1 nRT2 P1V1 = P2 V2 => P1V1 P2V2 = nRT1 nRT2 (Boyle’s Law) 5 - 25 Boyle’s Law Problem A gas tank holds 2750 L of nitrogen at 920 mm Hg. If the temperature remains constant, what will be the volume of nitrogen at standard pressure? P1 = 920 mm V1 = 2750 L T1 = T 2 P2 = 760 mm Hg V2 = ? T2 = T1 5 - 26 . P1V1 = P2V2 P1V1 V2 = P2 V2 = 920 mm × 2750 L = 3300 L N2 760 mm 5 - 27 Avogadro’s Hypothesis and Law Avogadro’s hypothesis says that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. Alternatively, 1.00 mole of any gas at STP (0°C, 1.00 atm) occupies a volume of 22.4 L. 5 - 28 Avogadro’s law says that the volume of a gas maintained at constant temperature and pressure is directly proportional to the number of moles of gas. 5 - 29 Deriving Avogadro’s Law Avogadro’s law can be derived from the ideal gas law. Assume the pressure and temperature remain constant. 5 - 30 Applying the IGL at the two different volumes yields: P1V1 = n1RT1 P2V2 = n2RT2 Dividing one equation by the other yields: P1V1 P2V2 = V1 V2 = n1 n2 n1RT1 n2RT2 => P1V1 P2V2 = n1RT1 n2RT2 (Avogadro’s Law) 5 - 31 General Gas Law The general gas law (combined gas law) is used when you are not restricting the IGL to only two variables (P, V or V, T). In most instances, two of the three physical properties of a gas are subject to change. The general or combined gas law can be derived from the ideal gas law. 5 - 32 Assume the same gas has existed at two different temperatures while only the number of moles of gas have remained constant. Mr. Bean has run out of gas! 5 - 33 Applying the IGL at two different pressures, temperatures, and volumes yields: P1V1 = nRT1 P2V2 = nRT2 Dividing one equation by the other yields: P1V1 P2V2 = nRT1 nRT2 P1V1 P2 V2 = T1 T2 => P1V1 P2V2 = nRT1 nRT2 (General Gas Law) 5 - 34 General Gas Law Problem What volume will 4.50 L of SO2 at -27°C and 600. mm Hg occupy if the temperature changes to 40.°C and the pressure to 700. mm Hg? P1 = 600. mm Hg V1 = 4.50 L T1 = -27°C = 246 K P2 = 700. mm V2 = ? T2 = 40.°C = 313 K 5 - 35 P1V1 = T1 P2V2 T2 600. mm × 4.50 L × 313 K V2 = 246 K × 700. mm V2 = 4.91 L SO2 5 - 36 Dalton’s Law of Partial Pressures The total pressure of a mixture of gases equals the sum of the pressures that each gas would exert if it was by itself. Each gas of behaves independently of the others. PT = P1 + P2 + … Pn where n = total number of gases 5 - 37 To determine the pressure of one of the gases present in the mixture, the mole fraction is used. Mole fraction, X, is a dimensionless number (no unit) which expresses the ratio of the number of moles of a particular component to the total number present in the mixture. n1 P1 = X1 × PT and X1 = n2 5 - 38 Dalton’s Law of Partial Pressures A 195 cm3 sample of helium is collected over water at 16°C and 97.5 kPa. What volume would the dry gas occupy at 117 kPa and 28°C? P1 = 97.5 kPa V1 = 195 cm3 T1 = 16°C = 289 K P2 = 117 kPa V2 = ? T2 = 28°C = 301 K 5 - 39 PT = PHe + PW PHe = PT – PW PHe = 97.5 kPa – 1.81 kPa = 95.7 kPa PW is the vapor pressure of water at 16°C which can be found in a table of vapor pressures of water. It is important to note that the total pressure, 97.5 kPa, is the sum of the water vapor pressure and the pressure of helium. 5 - 40 P1V1 = T1 P2V2 T2 97.5 kPa × 195 cm3 × 301 K V2 = 289 K × 117 kPa V2 = 1.70 × 102 cm3 He 5 - 41 Molar Volume Not At STP What is the density of argon at 37°C and 103.7 kPa? P1 = 101.3 kPa V1 = 22.4 dm3 T1 = 0°C = 273 K P1V1 T1 = P2 = 103.7 kPa V2 = ? T2 = 28°C = 310 K P2V2 T2 5 - 42 . V2 = 101.3 kPa × 22.4 dm3 × 310 K 273 K × 103.7 kPa V2 = 24.8 dm3 M = D V 39.95 g 3 = D = 1.62 g/dm 24.6 dm3 5 - 43 Gas Stoichiometry What mass of water is produced when 625 dm3 of hydrogen gas measured at 28°C and 97.3 kPa is burnt in the presence of oxygen as shown in the following reaction. 2H2(g) + O2(g) → 2H2O(g) P1 = 97.3 kPa V1 = 625 dm3 T1 = 27°C = 301 K P2 = 101.3 kPa V2 = ? T2 = 0°C = 273 K 5 - 44 . P1V1 P2V2 = T1 T2 V2 = 97.3 kPa × 625 dm3 × 273 K 301 K × 101.3 kPa V2 = 544 dm3 H2 STP, standard temperature and pressure, is 101.3 kPa and 0°C. 5 - 45 . m = 544 × dm3 H2 × 18.02 g H2O 2 mol H2O 1 mol H2 × 3 22.4 dm H2 2 mol H2O 2 mol H2 = 438 g H2O Remember that Avogadro’s hypothesis states that 1 mol of any gas occupies a volume of 22.4 L (or 22.4 dm3) at STP. This is the reason that the volume of the hydrogen had to be determined at 0°C before doing the stoichiometry. 5 - 46 More Gas Stoichiometry If 528 g of carbon disulfide react with oxygen, what volume of sulfur dioxide will be formed at 29°C and 98.2 kPa? CS2(l) + 3O2(g) → CO2(g) + 2SO2(g) m = 528 g CS2 5 - 47 V = 528 g CS2 × × 22.4 L SO2 1 mol SO2 P1 = 101.3 kPa V1 = 311 L SO2 T1 = 0°C = 273 K 1 mol CS2 × 76.13 g CS2 2 mol SO2 1 mol CS2 = 311 L SO2 P2 = 98.2 kPa V2 = ? T2 = 29°C = 302 K 5 - 48 P1V1 P2V2 = T1 T2 V2 = 101.3 kPa × 311 L × 302 K 273 K × 98.2 kPa V2 = 355 L SO2 Starting with 528 g CS2, you determine the volume of SO2 produced at 0°C, but the problem asked for the volume at 29°C. 5 - 49 Effusion and Diffusion According to Kinetic Molecular Theory (KMT), the average kinetic energy of gas molecules is given by KE = ½mv2 Because the KE determines the temperature of a substance, the molecules of hydrogen gas have the same KE as those of Kr at the same temperature. 5 - 50 A consequence of this is that the H2 molecules must be moving faster than those of Kr to compensate for their smaller mass. Two different types of molecular motion are diffusion and effusion. Diffusion is the spreading out of one substance throughout a second substance. An example of this is opening a bottle of ammonia (NH3). 5 - 51 Effusion is the escape of gas molecules through a tiny hole into evacuated space. To quantitatively compare gases, Graham’s law is used. Graham’s law is given by: r1 r2 = MM2 MM1 where r1 and r2 are the rates of effusion and the MM’s are the molar masses. 5 - 52 Graham’s law states that the rate of effusion of gas molecules is inversely proportional to the square root of their masses. This means that a lighter gas such as H2 effuses quicker than a more massive gas like Ar. 5 - 53 Graham’s Law Problem Compare the rates of effusion of hydrogen and krypton. r1 = H2 MM1 = 2.02 g•mol-1 r1 r2 = MM2 MM1 = r2 = Kr MM2 = 83.80 g•mol-1 83.80 g•mol-1 2.02 g•mol-1 = 6.44 H2 effuses 6.44 times faster than Kr. 5 - 54 Real Gases Ideal gases require two conditions: The gas molecules themselves do not occupy any volume. There are no intermolecular attractions. Real gases start to approach these ideal conditions at low pressures and high temperatures. 5 - 55 Real gases contradict these conditions because: the volume available to the molecules making up a real gas is less than the volume of the container because the molecules take up some space. the pressure exerted on the sides of the container is diminished because the intermolecular attractions result in fewer collisions with the sides of the container. 5 - 56 van der Waal’s Equation (Pobs + a(n/V)2) × (V – nb) = nRT The first term involving the Pobs is the corrected pressure factor. This correction is necessary because of the intermolecular attractions. As a result of these attractions, the observed pressure will be smaller than predicted from the ideal gas law. 5 - 57 The second term, V – nb, is the corrected volume which will be smaller than the container volume because the gas molecules themselves have volume. There will be less free space for the molecules to move about. The constants a and b are called empirical constants because their value is determined by fitting the equation to the experimental results. 5 - 58 The values for a and b are different for different molecules and can be found in a table of physical constants. 5 - 59 van der Waals Problem Calculate the pressure that H2O will exert at 50°C if 1.00 mol occupies 32.0 L assuming H2O obeys the van der Waals equation. The constants for H2O are: L2•atm a = 5.46 mol2 T = 50°C = 323K b = 0.0305 V = 32.0 L L mol n = 1.00 mol 5 - 60 (Pobs + a(n/V)2) × (V – nb) = nRT . 2•atm L 2) (1.00 mol/32.0 L) (Pobs + 5.46 × mol2 × 32.0 L – 0.0305 L mol = 0.0821 L•atm × 323 K = 0.829 atm mol•K 5 - 61