Energy, Liquids, Solids: Chemistry Course Materials
advertisement

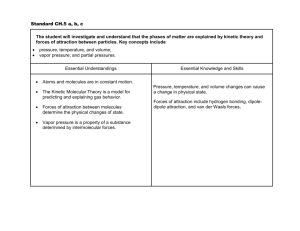
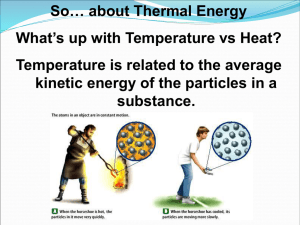
Chapter 10: Energy Chapter 14: Liquids and Solids Purpose Energy can be transferred or it can change form, but it cannot be created or destroyed. Matter can be described by its physical properties. The physical properties of a substance depend on the spacing between the particles of the substance and the forces of attraction between the particles. Heating curves and phase diagrams graphically relate temperature, energy, phase of matter, and strength of intermolecular forces. A and B 3/17 and 3/18 In-Class Chapter 10 & 14 Notes 3/19 and 3/20 Go over Chapter 10 &14 Worksheet #1 Specific Heat Lab Go over Chapter 10 & 14 Worksheet #2 Food Energy Lab Go over Chapter 10 & 14 Review Chapter 10 & 14 Test 3/23 and 3/24 3/25 and 3/26 HW Assignments Chapter 10 & 14 Worksheet #1 Chapter 10 & 14 Worksheet #2 Chapter 10 & 14 Review Vocabulary energy thermodynamics temperature kinetic energy potential energy heat endothermic exothermic enthalpy heat of reaction entropy calorie Joules specific heat capacity calorimeter Hess’s Law intermolecular forces intramolecular forces dipole-dipole hydrogen bonding London dispersion forces heat of vaporization heat of fusion sublimation evaporation condensation triple point critical point deposition melting freezing By the end of this Topic, you should be able to demonstrate proficiency in the following areas: Essential Understandings The phase of a substance depends on temperature and pressure. Forces of attraction (intermolecular forces) between molecules determine their state of matter at a given temperature. Forces of attraction include hydrogen bonding, dipole-dipole attraction, and London dispersion (van der Waals) forces. Solid, liquid, and gas phases of a substance have different energy content. Pressure, temperature, and volume changes can cause a change in physical state. Specific amounts of energy are absorbed or released during phase changes. A heating/cooling curve graphically describes the relationship between temperature and energy (heat). It can be used to identify a substance’s phase of matter at a given temperature as well as the temperature(s) at which it changes phase. It also shows the strength of the intermolecular forces present in a substance. Molar heat of fusion is a property that describes the amount of energy needed to convert one mole of a substance between its solid and liquid states. Molar heat of vaporization is a property that describes the amount of energy needed to convert one mole of a substance between its liquid and gas states. Specific heat capacity is a property of a substance that tells the amount of energy needed to raise one gram of a substance by one degree Celsius. The value of these properties are related to the strength of their intermolecular forces. Essential Knowledge, and Skills In order to meet this standard, it is expected that students will distinguish between an endothermic and exothermic process. identify how hydrogen bonding in water plays an important role in many physical, chemical, and biological phenomena. graph and interpret a heating curve (temperature vs. time) interpret a phase diagram of water. calculate energy changes, using specific heat capacity. explain the relationship between intermolecular forces and relative size of heat of fusion/vaporization. calculate the energy changes, using molar heat of fusion and molar heat of vaporization. SOL Standards CH.5 The student will investigate and understand that the phases of matter are explained by kinetic theory and forces of attraction between particles . Key concepts include d) phase changes; e) molar heats of fusion and vaporization; f) specific heat capacity;