vapor pressure
advertisement

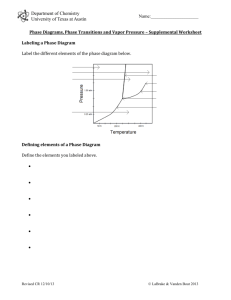
• A change in state is called a phase change • Evaporation is the change in state from liquid to gas • Sublimation is the change from solid to gas • Both deal with the motion of molecules • You have also probably noticed that the evaporation of liquids produce a cooling effect Molecules that are able to escape from the liquid have kinetic energies larger than the average. When they leave, the average kinetic energy of the remaining molecules is less, so the temperature is lower. • The rate of evaporation depends on the temperature, surface area, and strength of the intermolecular attractions At higher temperature, the total fraction of molecules with kinetic energy large enough to escape is larger so the rate of evaporation is larger. • For a given liquid, the rate of evaporation per unit surface area is greater at a higher temperature Kinetic energy distribution in two different liquids, A and B, at the same temperature. The minimum kinetic energy required by molecules A to escape is less than for B because the intermolecular attractions in A are weaker than in B. This causes A to evaporate faster than B. • As soon as a liquid is placed in an empty container, it begins to evaporate • Once in the gas phase, molecules can condense by striking the surface of the liquid and giving up some kinetic energy • The rate of evaporation equals the rate of condensation at equilibrium • This can occur in a system where the molecules are constrained to remain close to the liquid surface (a) The liquid begins to evaporate in the closed container. (b) Dynamic equilibrium is reached when the rate of evaporation and condensation are equal. • Similar equilibria are reached in melting and sublimation At the melting point a solid begins to change into a liquid as heat is added. As long no heat is added or removed melting (red arrows) and freezing (black arrows) occur at the same rate an the number of particles in the solid remains constant. At equilibrium, molecules evaporate from the solid at the same rate as molecules condense from the vapor. • When molecules evaporate, the molecules that enter the vapor phase exert a pressure called the vapor pressure • The equilibrium vapor pressure is the vapor pressure once dynamic equilibrium has been reached • The equilibrium vapor pressure is usually referred to as simply the vapor pressure • Vapor pressures can be measured using a manometer • Measuring the (equilibrium) vapor pressure of a liquid at a specific temperature Variation of vapor pressure with temperature. Ether is said to be volatile because it has a high vapor pressure near room temperature. • Volume changes can effect vapor pressure (a) Equilibrium exists between liquid and vapor. (b) The volume is increased, the pressure drops, and the rate of condensation drops. (c) Once more liquid evaporates, equilibrium is reestablished and the vapor pressure returns to its initial value. • Solids also have vapor pressures • At a given temperature, some of the particles at the solid have enough kinetic energy and escape into the vapor phase • When vapor particle collide with the surface, they can be captured • The pressure of the vapor that is in equilibrium with the solid is called the equilibrium vapor pressure of the solid • The boiling point of a liquid can be defined as the temperature at which the vapor pressure of the liquid is equal to the prevailing atmospheric pressure • The normal boiling point is the temperature at which the vapor pressure is 1 atm • Molecules with higher intermolecular forces have higher boiling points Boiling points of the hydrogen compounds of elements of Groups IVA, VA, VIA, and VIIA of the periodic table. The boiling points of molecules with hydrogen bonding are higher that expected. • Heating and cooling curves can be used to determine melting and boiling points (a) A heating curve observed when heat is added at a constant rate. (b) A cooling curve observed when heat is removed at a constant rate. The “flat” regions of the curves identify the melting and boiling points. Supercooling is seen hear as the temperature of the liquid dips below its freezing point. • The energy associated with the phase changes can be expressed per mole • The molar heat of fusion is the heat absorbed by one mole of solid when it melts to give a liquid at the same temperature and pressure • The molar heat of vaporization is the heat absorbed when one mole of the liquid is changed to one mole of vapor at constant temperature and pressure • The molar heat of sublimation is the heat absorbed by one mole of a solid when it sublimes to give one mole of vapor at constant temperature and pressure • All of these quantities tend to increase with increasing intermolecular forces • The concept of equilibrium is important and will be encountered again • Equilibria are often disturbed or upset • According to Le Chatelier’s Principle – When a dynamic equilibrium in a system is upset by a disturbance, the system responds in a direction that tends to counteract the disturbance and, if possible, restore equilibrium • The term position of equilibrium is used to refer to the relative amounts of the substance on each side of the double (equilibrium) arrows • Consider the liquid vapor equilibrium liquid heat vapor • Increasing the temperature increases the amount of vapor and decreases the amount of liquid • We say that the equilibrium has shifted • This can also be referred to as a right shift because more vapor is produced at the expense of the liquid • Temperature-pressure relationships can be represented using a phase diagram The phase diagram of water. The line AB is the vapor pressure curve for ice; BD the vapor pressure curve for liquid water; BC the melting point line; point B the triple point (the temperature where all three phases are in equilibrium); and point D labels the critical point (and the critical temperature and pressure). Above the critical temperature a distinct liquid phase does not exist, regardless of pressure. • A substance that has a temperature above its critical temperature and a density near its liquid density is called a supercritical fluid • Supercritical fluids have some unique properties that make them excellent solvents • The values of the critical temperature tends to increase with increased intermolecular attractions between the particles