File
advertisement

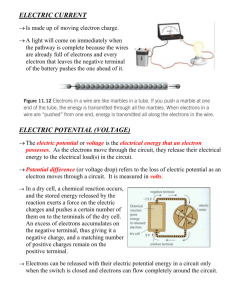
Term 332 EE2010: Signals and Systems Analysis 2. Introduction Dr. Mujahed Al-Dhaifallah EE2010_Lecture2 Al-Dhaifallah_Term332 1 Dr. Mujahed Al-Dhaifallah مجاهد آل ضيف هللا.د Office: Dean Office. E-mail: muja2007hed@gmail.com Telephone: 7842983 Office Hours: SMT, 1:30 – 2:30 PM, or by appointment EE2010_Lecture2 Al-Dhaifallah_Term332 2 Rules and Regulations No make up quizzes DN grade == 25% unexcused absences Homework Assignments are due to the beginning of the lectures. Absence is not an excuse for not submitting the Homework. EE2010_Lecture2 Al-Dhaifallah_Term332 3 Grading Policy Exam 1 (10%), Exam 2 (15%) Final Exam (60%), Quizzes (5%) HWs (5%) Attendance & class participation (5%), penalty for late attendance Note: No absence, late homework submission allowed without genuine excuse. EE2010_Lecture2 Al-Dhaifallah_Term332 4 Attendance Regular lecture attendance is required. There will be part of the grade on attendance If you missed any class or tutorial, you are still responsible for anything you miss—announcements, quizzes, etc. Quizzes Announced After each HW. From HW material Assignment Requirements Late assignments will not be accepted. assignments are due at the beginning of lecture. Sloppy or disorganized work will adversely affect your grade. Exams Attendance is mandatory. Make-up exam are not given unless a valid, documented emergency has arisen Homework Send me e-mail Subject Line: “EE 2010 Student” The Course Goal The aim of this course is to provide an understanding of the fundamentals and analysis of electric circuits. EE2010_Lecture2 Al-Dhaifallah_Term332 10 Course Objectives After successfully completing the course, the students will be able to 1. Understand the fundamental concepts of electric circuits. 2. Understand the main circuit elements including energy storage elements. 3. Learn the different circuit analysis techniques. 4. Obtain the equivalent circuits and find out the conditions of maximum power transfer. 5. Apply analysis techniques to sinusoidal circuits. 6. Evaluate the power in sinusoidal circuits. EE2010_Lecture2 Al-Dhaifallah_Term332 11 Textbooks Introductory Circuit Analysis Robert Boylestad Course Syllabus 1. Introductory material: Introduction 2. Basic circuit elements and concepts: Current, Voltage, Resistance. Chapters (2 and 3) 3. Basic laws of circuit theory: Ohm's law, Power and Energy. Devices: Battery, Power Supply, Multi-meters, Circuit Breakers (Chapter 4) 4. Series Circuits, Kirchhoff's Voltage law. (Chapter 5) EE2010_Lecture2 Al-Dhaifallah_Term332 13 Course Outlines 4. Parallel Circuits, Kirchhoff's Current law (Chapter 6) 5. Series - Parallel Circuits. (Chapter 7) 6. Techniques of circuit analysis: Source transformation, nodal and mesh analysis. (Chapter 8) 7. Circuit theorems: superposition principle, Thevenin and Norton theorems; maximum power transfer theorem. (Chapter 9) EE2010_Lecture2 Al-Dhaifallah_Term332 14 Course Outlines 6. Capacitors, Inductors, Series and Parallel connection. (Chapters 10 and 12) 7. Sinusoidal Source, Complex Numbers, Frequency Domain (Phasor) Circuit. (Chapters 13 and 14). EE2010_Lecture2 Al-Dhaifallah_Term332 15 Current, Voltage and Resistance EE 2010: Fundamentals of Electric Circuits Mujahed AlDhaifallah Atoms and their structure electron neutron proton Atomic Structure Mass of an Electron = 9.11 x 10-28 gm. Mass of a Proton = 1.672 x 10-24 gm. Proton is ~1836 times heavier than the electron Atomic Structure Unit of Charge = Coulombs Charge on electron = charge on a proton = 1.6 x 10-19 C 1 Coulomb = Charge on 6.242 x 1018 electrons Coulomb’s Law Like charges repel, opposites attract F = k Q1 Q2 / r2 k = 9 x 109 (units?) Coulomb’s Law Like charges repel, opposites attract F = k Q1 Q2 / r2 K = 9 x 109 N m2/C2 Conduction In metals, the electrons are “more free” than the insulators. Whenever there is a charge present at one end, the electrons flow to (or away) from that charge. Current Rate of flow of charge 1 Amp = 1 Coulomb / 1 Second. Question If a laptop constantly needs 2 Amps current from a battery, how many electrons are drained from the battery in one hour? 1 Amp = 6.242 x 1018 electrons/second 2 Amp = 12.484 x 1018 electrons/second In one hour - > 3600 x 12.484 x 1018 electrons Answer is 4.49 x 1022 electrons Question What’s the weight of all those electrons? 4.49 x 1022 x 9.11 x 10-28 gm 4.09 x 10-5 gm Equations I = Q/ t Q=Ixt t = Q/I Examples Find the current in amperes if 650 C of charge pass through a wire in 50 s. If 465 C of charge pass through a wire in 2.5 min, find the current in amperes. If a current of 40 A exists for 1 min, how many coulombs of charge have passed through the wire? EE2010_Lecture2 Al-Dhaifallah_Term332 27 Example Consider the plot of net positive charge moving past a point shown in Fig. Over the time interval 1 s ≤ t ≤ 3 s. Find i(t) EE2010_Lecture2 Al-Dhaifallah_Term332 28 Potential Every particle of mass m raised to a height h above the earth’s surface has a potential energy m.g.h This potential energy can be raised by raising the particle a little higher When the particle is set free, it travels to the point of least potential. Electric Potential Similarly, a charge wants to travel to a lower “electric” potential. A negative charge on the other hand, wants to travel to a higher potential. Each point in a circuit has a potential. Voltage Voltage is always measured between two points. It is defined as the difference of potential between the two points. Measured in volts Volts 1 volt is defined as the potential difference, which results in an energy exchange of 1 Joule due to the movement of 1 Coulomb across it. DC Voltage Supply Conductivity Copper is the most popular conductor. Metal Conductivity (%) Silver 105 Copper 100 Gold 70.5 Aluminum 61 Tungsten 31.2 Nickel 22.1 Iron 14 Constantan 3.52 Nichrome 1.73 Calorite 1.44 Resistance Resistance is proportional to length length direction of current flow Resistance Resistance is inversely proportional to the cross sectional area direction of current flow Resistance R = ρ L/A ρ is the resistivity of the material (units?) Material ρ (10-8 Ohm-Metres) Silver Copper Gold 1.645 1.723 2.443 Aluminum Tungsten 2.825 5.485 Nickel Iron 7.811 12.299 Tantalum Nichrome 15.54 99.72 Tin Oxide Carbon 250 3500 Color Coding 5 Bands of code (3 are mandatory) Bands 1 - 3 the value of the resistor Band 4 the range (tolerance) Band 5 the reliability Color Code (Band 1-3) Color Value Black 0 Brown 1 Red 2 Orange 3 Yellow 4 Green 5 Blue 6 Violet 7 Gray 8 White 9 Example 2 6 x 103 = 26 K Ohms Band 3 (special cases) Gold = 0.1 Red Blue Gold = 2.6 Ohm Silver = 0.01 Red Blue Silver = 0.26 Ohm More Bands Band 4 Tolerance Gold 5% Silver 10% None 20% Band 5 Reliability (after 1000 Hrs of use) Brown 1% Red 0.1% Orange 0.01% Yellow 0.001% Example = 26 K Ohms ± 5%, 1 in 100,000 fails after 1000 hrs of use