IL-12
advertisement
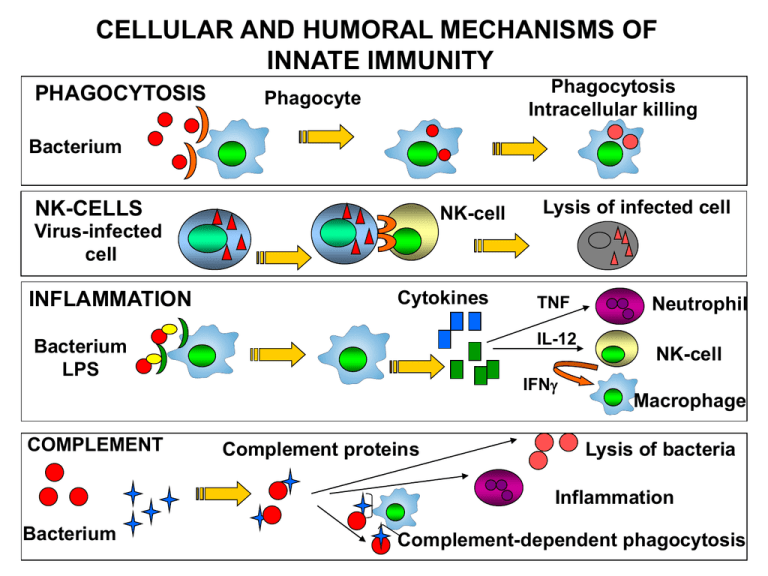
CELLULAR AND HUMORAL MECHANISMS OF INNATE IMMUNITY PHAGOCYTOSIS Phagocytosis Intracellular killing Phagocyte Bacterium NK-CELLS NK-cell Lysis of infected cell Virus-infected cell INFLAMMATION Cytokines IL-12 Bacterium LPS COMPLEMENT TNF IFN Complement proteins Neutrophil NK-cell Macrophage Lysis of bacteria Inflammation Bacterium Complement-dependent phagocytosis DEVELOPMENT OF MACROPHAGES Stem cell Bone marrow Szerv/szövet Makrofág populáció Bone PU-1 Osteoclast Central nervous system Microglia Connective tissue Placenta Histiocytes Hofbauer cells Vessel Monocyte Kidney Liver Peritoneum Tissues organs Macrophage Mesengial cells Kupffer cells Peritoneal macrophages Lung Alveolar macrophages Skin Epidermal and dermal macrophages Macrophages can act as stromal cells to help the differentiation of other cells. RECEPTORS AND OTHER MOLEKULES OF MACROPHAGES LPS receptor (CD14) + TLR4 Scavanger receptor Mannose receptor MHCI TLR – pathogen pattern FcRI (CD64) Ag + IgG complex FcRII (CD32) FcRIII (CD16) LFA1 (CD11a/CD18) peroxidáz hidroláz MHCII CR1 (CD35) CR3 (CD11b/CD18) Activation of macrophages Dale, D. C. et al. Blood 2008;112:935-945 Copyright ©2008 American Society of Hematology. Copyright restrictions may apply. Receptors and molecules of macrophages RECEPTOR LIGAND FUNCTION FcR IgG, IgE Opsonized phagocytosis, ADCC, release of inflammatory mediators CR3 iC3B, ICAM-1 Opsonized phagocytosis Macrophage Mannose Receptor Lectin, Endocytosis, phagocytosis, antigen capture and transport SR-A LPS, polianions, lipoteikolic acid Endocytosis, phagocytosis, adhesion CD14 LPS Transduces LPS aktivation , TNFa release CCR1 MIP1a, MCP-3 Recruitment, migration of monocytes CCR3 Eotaxin Haematopoiesis, HIV-1 coreceptor CCR5 MIP1 Haematopoiesis, HIV-1 coreceptor CXCR4 SDF-1a Haematopoiesis, HIV-1 coreceptor Activation of macrophages Activation of macrophages Inflammatory cytokines Microorganism TNF IL-6 IL-12 Inflammatory cytokines Antimicrobial substances IL-12 IL-18 Th 1 cell NK cell IFN Inactivation IL-4 IL-10 T cell APC IL-13 Th 2 cell Alternative activation: Mannose receptor – endocytosis Th2 chemokines NOS inhibition Tissue regeneration Functions of activated macrophages in anti-bacterial immunity Macrophage Response** Role in Cell-mediated Immunity Production of reactive oxygen intermediates, nitric oxide; increased lysosomal enzymes Killing of microbes in phagolysomes (effector function of macrophages) Secretion of Cytokines (TNF-a, IL-1, IL-12) TNF-a, IL-1: leukocyte recruitment (inflammation) IL-12: TH-1 differentiation, IFN- production (induction of response) Increased expression of: CD80, CD86 Class I, Class II MHC Increased T cell activation (amplification) ** These macrophage responses are induced by CD40 ligation to CD154 (CD40L) and T cellderived IFN- in cell-mediated immunity; similar responses are induced by microbial products, particularly LPS, and NK cell-derived IFN- in innate immunity. Phagocytosis Intracellular Bacterial Killing Intracellular bacterial killing Reactive oxygen species Intracellular bacteria in phagolysosomes are susceptible to reactive oxygen species, which damage cell wall components and fragment genomic DNA. Reactive Oxygen Intermediate (ROI) production is initiated by membranebound NADPH oxidase, which is activated by IFN-. NADPH Oxidase O2 + NADPH NADP + O2- + H+ O2- is further metabolized by superoxide dismutase (SOD). SOD - O2 + H+ O2 + H2O2 Intracellular bacterial killing Reactive oxygen species In the presence of appropriate iron catalysts, the Haber-Weiss reaction takes place: O2- + Fe3+ H2O2 + Fe2+ O2- + H2O2 O2 + Fe2+ OH + OH- + Fe3+ OH +OH- + O2 O2- is transformed into 1O2 and OH are short-lived, powerful oxidants with high antibacterial activity, causing damage to DNA, membrane lipids, and proteins. Nomenclature O2- - superoxide anion OH – hydroxyl radical containing a free electron 1O – singlet oxygen, a highly reactive form of O 2 2 Intracellular bacterial killing Reactive oxygen species myeloperoxidase-dependent killing The lysosomes of granulocytes and monocytes/macrophages contain the enzyme myeloperoxidase (MPO). This enzyme catalyzes the following reaction: H2O2 + Cl- MPO OCl- + H2O Hypochlorous acid and chloramines are formed – both agents further increase the bactericidal power of the ROI system by destroying biologically important proteins through chlorination (Halogenation). Dale, D. C. et al. Blood 2008;112:935-945 Copyright ©2008 American Society of Hematology. Copyright restrictions may apply. Intracellular bacterial killing Reactive nitrogen species Phagocytes possess an additional pathway for generating reactive species that possess bactericidal activity. These species are the reactive nitrogen intermediates (RNI). The principal RNI is nitric oxide (NO), which is derived from the terminal guanidino-nitrogen atom of L-arginine. The reaction is catalyzed by the inducible form of nitric oxide synthase (iNOS; NOS2), leading to the formation of L-citrulline and NO. Intracellular bacterial killing Reactive nitrogen species NO can act as an oxidizing agent alone, or it interacts with O2- to form unstable peroxynitrite (ONOO-). This may be transformed to the more stable anions, NO2- and NO3-, or decomposed to NO. O2- + NO ONOO- ONOO- + H+ NO2- + .OH NO2- + .OH NO3- + H+ ONOO- + H+ .OH + NO. NO· and ONOO- are highly reactive antimicrobial agents. NO· may be transformed to nitrosothiols expressing the most potent antimicrobial activity. In contrast, NO2- and NO3- are without notable effects on microorganisms. Effects of reactive species O2iNOS 0NO2- NO Destruction of mitochondria DNA destruction Protein destruction S-nitrosilation PARP activation Producing ROI 1 NAD ADP – ribose + NAM Decreased energy 4 ATP CELL DEATH Detection of mediators produced by macrophages Phagocytosis assay: yeast,uptake of fluorescent beads, Preopsonized FITC labeled E. coli (FACS) NO : sera, (clinical) Gries Ilosvay assay (reduction of nitrit and nitrate to NO), Arginin –Citrulin reaction, detection of iNOS activity (IHC, Western blot), measure of released NO by DAF (FACS) Citokines : TNFa, TGFb (ELISA, ELISPOT) ROI: NBT reduction assay Hydrogen peroxide assay Citochrome c reduction assay Intracellular bacterial evasion of killing in phagocytes Intracellular bacteria have evolved strategies to evade killing by the mechanisms available to the phagocyte. Macrophage effector capacity Microbial evasion mechanism Defensins Unknown Phagosome acidification Phagosome neutralization Phagosome–lysosome fusion Lysosomal enzymes Inhibition of phagosome–lysosome fusion Resistance against enzymes Intraphagolysosomal killing Evasion into cytosol Robust cell wall C3b receptor-mediated uptake, ROI ROI detoxifiers, ROI scavengers RNI Unknown (ROI detoxifiers probably interfere with RNI) Iron starvation Microbial iron scavengers (e.g., siderophores) Tryptophan starvation Unknown Granulomatosus inflammation - chronic inflammation - epitheloid cells in the infiltrate, these are modified macrophages whit pale cytoplasm and nucleus - cells with no intercellular substances (epithelial cell-like tight connections) - cells become multinucleated Langhans type giant cells ↓ Granulomatosus inflammation: granuloma formation with cell death Granulomas syncytium (multinucleated giant cells) lymphocytes Periapical granuloma = dental granuloma Modified granulation tissue containing elements of chronic inflammation, located adjacent to the root apex of a tooth with infected, necrotic pulp. Tuberculosis (TBC) Phatogen: Mycobacterium tuberculosis Mycobacterium bovis, Mycobacterium africanum, Mycobacterium canetti, and Mycobacterium microti can also cause tuberculosis, but these species do not usually infect healthy adults Tuberculosis most commonly attacks: • the lungs (as pulmonary TB) •central nervous system (meningitidis) • lymphatic and circulatory system (miliary TB) •genitourinary system, •bones, joints • skin From 2000 to 2004, 20% of TB cases being resistant to standard treatments and 2% resistant to second-line drugs. 2.000.000.000 infected worldwide Mycobacterium infection 3. T cell and macrophage activation IFN- TNF 2. Antigen presentation IL-12 1.Infection of macrophages Macrophage CD4+ T cell Macrophage DS CD8+ T cell Macrophage perforin granulysin Mycobacterium infection Symptom-free carriers 90% IFN- TNF IL-12 CD4+ T cell MTb. remains in granulomas Macrophage DS Macrophage Healing (?) CD8+ T cell perforin granulysin Reactivation (10%) Reinfection HIV infection: 800x more tuberculosis Acute tuberculosis - 10% (HIV infection) Other immune suppression Dissemination Transmission Appearance and frequency of TBC The 90% of people infected with bacteria are symptome-free, living with latent TBC (LTBI), their opportunity is 10% to develop disease. Without treatment, 50% of TBC diseases are lethal. TBC is one of the three most dangerous infectious diseases worldwide, mortality is two times higher than to malaria. 2.000.000.000 infected persons Morbus hungaricus Morbidity of TBC in Hungary: • • • first decades of the later century: 340-380/100000 citizens 1955: 30/100000 citizens (lower than the European average!) reason: regular screening, vaccination, up-to-date therapy In last years: increasing numbers of TBC reason: optimistic attitude, ease of strict control Skin and bone - tuberculosis Primary infection Miliaris tuberculosis CNS tuberculosis Lymphnode tuberculosis Diagnostic testing of tuberculosis Tuberculin skin test(TST) Most often applied tuberculin test: Mantoux’s test PPD (purified protein derivative) Size of induratio (after 48h) Disadvantages: • Not specific for M. tuberculosis • Positiv reaction: in case of atypical Mycobacterial diseases and BCG vaccination also Diagnostic testing of tuberculosis IFNγ release assay (IGRA) - ELISPOT ESAT-6 (early secrete antigen target 6) and CFP-10 (culture filtrate protein) stimulatory antigens Measuring: release of IFNγ by T cells Results: SFU (Spot Forming Unit) Advantages: • More specific than TST • Can be repeated • The testing protocol requires only one visit Disadvantages: Reversion: a previously positive IGRA results becomes negative upon revers testing, due to • clearing of TB infection (spontaneous or due to treatment) • biological variations among IGRA+ individuals • the life cycle of M. tuberculosis, where the Mycobacterium enters a dormant state in which it may not be secrete ESAT-6 and CFP-10 antigens (but instead secrete other antigens which are not used in currently available IGRAs) Treatment First line tuberculosis drugs 3-letter 1-letter Drug EMB E Ethambutol INH H Isoniazid PZA Z Pyrazinamide RMP R Rifampicin STM S streptomycin Second line tuberculosis drugs CIP (none) Ciprofloxacin MXF (none) Moxifloxacin PAS P p-aminosalicylic acid All first-line anti-tuberculous drug names have a standard three-letter and a single-letter abbreviation: •Streptomycin is STM or S, •isoniazid is INH or H, •rifampicin is RMP or R, •ethambutol is EMB or E, •pyrazinamide is PZA or Z. The US commonly uses abbreviations and names that are not internationally recognised: rifampicin is called rifampin and abbreviated RIF; streptomycin is commonly abbreviated SM. The standard "short" course treatment for tuberculosis (TB): 2 months: isoniazid, rifampicin, pyrazinamide, and ethambutol + 4 months: isoniazid and rifampicin alone For latent tuberculosis, the standard treatment is six to nine months of isoniazid alone