Raleigh fractionation
advertisement
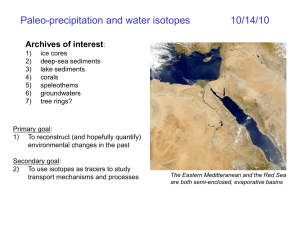
Stable Isotopes – Raleigh distillation and water isotopes 10/4/12 Lecture outline: 1) mass balance 2) Raleigh distillation 3) the hydrological cycle 4) D and 18O variability spectrometer light intake Chalk cliffs formed in Cretaceous Mass balance of stable isotopes Principle: stable isotopes are CONSERVED, unlike radioactive isotopes Therefore, if one reservoir is enriched, the other reservoir must be depleted Rt d R t 0 Ri di i R (reservoir size) is expressed in moles ‘d’ represents the delta value for a given reservoir, expressed in per mil Example: What was the glacial-interglacial sea level change? Given: G-I 18O change = +1.3‰ (SMOW) present-day ocean = 0‰ (SMOW) glacial ice caps averaged -35‰ Vo *0‰ Vi * 35‰ + Vgo *1.3‰ Vi Vo Vgo sea level = 140m Continual fractionation in a closed system: Raleigh distillation ex: rainfall from cloud original vapor enriched phase ( ? 1) equilibrium first drop enriched phase equilibrium next drop next vapor enriched phase equilibrium next drop final vapor enriched phase final drop rain becomes lighter TIME next vapor TIME vapor becomes lighter IF PRODUCT REMOVED (cannot re-equilibrate w/ parent liquid) NO FRACTIONATION FOR LAST DROP. . . Why? Raleigh distillation model We can track the progression of the vapor-rainfall if we know: 1. the initial isotopic ratio of the vapor 2. the fraction of vapor remaining liquid RV f 1 RV0 18O (‰ where RV is the isotopic ratio of the vapor RV0 is the initial isotopic ratio of the vapor f is the fraction of vapor remaining vapor α is the fractionation factor We can also derive the formula for the Rrain as a function of α: RR f 1 RV0 If the of vapor to liquid is 1.0092, what is the of liquid to vapor? After Dansgaard, 1964 NOTE: fractionation increasing because T(cloud) decreasing Raleigh distillation in the real world If the tropics are the source of all cloud moisture, then the 18O of rainfall _________ from equator to pole. What also happens as you move from equator to pole? This effect would ________ the 18O of rainfall at the poles. What other natural systems might be characterized by Raleigh fractionation? The Hydrosphere How do 18O, 16O (18O) and 2H, 1H (D) move through this system? Water Isotopic Variations Ocean 18O Lake Michigan 18O = -7‰ D = -54‰ Lake Chad 18O = -20‰ D = -110‰ Dead Sea 18O = +4.4‰ D = 0‰ = 0 ± 2‰ D = 0 ± 16‰ What processes explain these variations? NOTE: water isotopes are always reported with respect to SMOW Water Isotopic Fractionation – review from last lecture Reminder: Oxygen and hydrogen isotopes are strongly fractionated as they move through the hydrological cycle, because of the large fractionation associated with evaporation/condensation. This fractionation is temperature-dependent. GNIP – global network of isotopes in precipitation Rainwater samples are routinely collected for 18O and D analysis all over the world. The data are stored and managed by GNIP, and used to study the processes that fractionate water isotopes. Water Isotopic Fractionation – some data Rozanski, 1993 18O of rain near SMOW in tropics, highly depleted in high-latitudes 18O of rain decreases far from vapor source (Raleigh) and is heavier during winter (temperature) Temperature effect on the 18O of precipitation holds for both spatial T variability and temporal variability Rozanski, 1993 But what if we add all the GNIP global 18Oprecip data? A bit more complicated, but generally strong relationship. However, what is happening at higher temperatures? Rozanski, 1993 The so-called “amount” effect: more rain, heavier d18O NOTE: only in tropics (<30° N and S), where “deep convection” takes place Empirical relationship – meaning….? It would be difficult to explain a vapor source at +1‰, when the tropical oceans are ~0‰. Thought to be linked to increased evaporation of raindrop in dry, under-saturated environment… (i.e. vapor is -9‰ ish, but the raindrop is enriched as it falls from the sky) Dansgaard, 1964 Rozanski, 1993 Mechanism still unknown – need atmospheric modeler’s help. Surface Water Salinity-18O relationship - general d 18O 0.45* S 15.5 Global precipitation So 18O of surface waters, like salinity, is also correlated to evaporation – precipitation. Surface Water Salinity-18O relationship - tropics d 18O 0.273* S 9.4 Fairbanks et al., 1997 Slope of 18O-salinity relationship is 0.273 in the deep tropics (<5° N and S), vs. 0.45 elsewhere. Why? The “Global Meteoric Water Line” – what happens to 18O happens to D, but with a different annual mean dD vs. d18O of precipitation But month-to-month variations at a given site fall off this line – “deuterium excess” d d D 8* d 18O d D 8* d 18O 10 Craig, 1961 Rozanski, 1993 Why don’t all waters fall on the GMWL? Or…. why do different “source” waters have different ‘deuterium excess’ values? Fact: water vapor above the ocean is -13‰ in 18O, not the -9.2‰ expected from equilibrium fractionation. Why? Planetary boundary layer -evaporation not purely equilibrium process -what other type of fractionation is involved? 1-3km the layer where exchange occurs between the surface and the free atmosphere Water Given the potential for complicated boundary layer physics, it’s a wonder that the GMWL exists at all! Deuterium excess Humid regions will show smaller departures from GMWL than arid regions. Generally interpreted as a proxy for the “source” of the moisture. Modeling water isotopes in the hydrosphere Full atmospheric General Circulation Model (GCM) with water isotope fractionation included. Noone, D., 2002 Goal: quantify physical processes associated with water isotope variability Applications: atmospheric mixing, vapor source regions, impact of climate variability on hydrological cycle, interpretation of paleoceanographic records