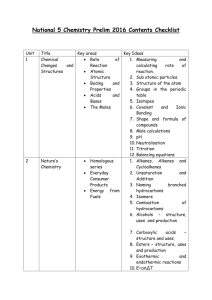
Year 10 Chemistry TRIPLE | Learning Cycle 2 | Medium Term Plan
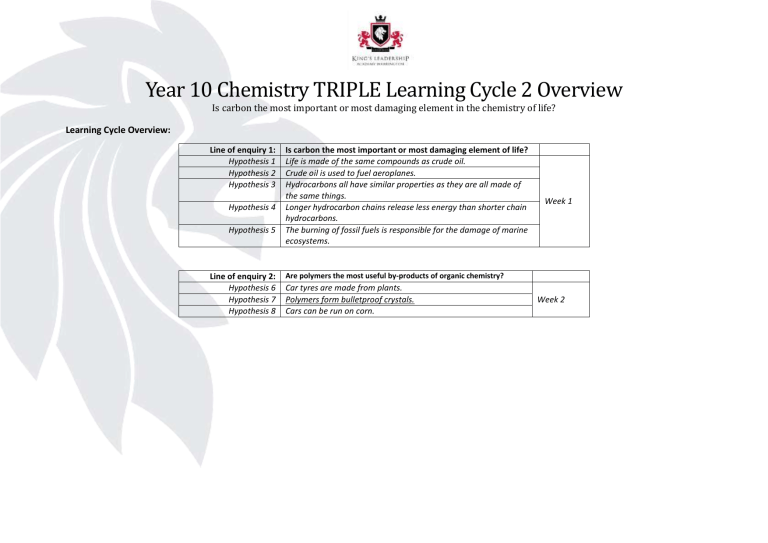
Year 10 Chemistry TRIPLE Learning Cycle 2 Overview
Is carbon the most important or most damaging element in the chemistry of life?
Learning Cycle Overview:
Line of enquiry 1: Is carbon the most important or most damaging element of life?
Hypothesis 1 Life is made of the same compounds as crude oil.
Hypothesis 2 Crude oil is used to fuel aeroplanes.
Hypothesis 3 Hydrocarbons all have similar properties as they are all made of the same things.
Hypothesis 4 Longer hydrocarbon chains release less energy than shorter chain hydrocarbons.
Hypothesis 5 The burning of fossil fuels is responsible for the damage of marine ecosystems.
Week 1
Line of enquiry 2:
Are polymers the most useful by-products of organic chemistry?
Hypothesis 6 Car tyres are made from plants.
Hypothesis 7 Polymers form bulletproof crystals.
Hypothesis 8 Cars can be run on corn.
Week 2
Year 10 Chemistry TRIPLE | Learning Cycle 2 | Medium Term Plan | Science 2015/16
Line of enquiry one: Is carbon the most important or most damaging element in the chemistry of life?
Intentions for learning from AQA:
Crude oil is a mixture of a very large number of compounds.
A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged. It is possible to separate the substances in a mixture by physical methods including distillation.
Most of the compounds in crude oil consist of molecules made up of hydrogen and carbon atoms only (hydrocarbons). Most of these are saturated hydrocarbons called alkanes, which have the general formula C n
H
2n+2.
Alkane molecules can be represented in the following forms: C
2
H
6
. Displayed formula.
The many hydrocarbons in crude oil may be separated into fractions, each of which contains molecules with a similar number of carbon atoms, by evaporating the oil and allowing it to condense at a number of different temperatures. This process is fractional distillation.
Some properties of hydrocarbons depend on the size of their molecules. These properties influence how hydrocarbons are used as fuels.
Hydrocarbons can be cracked to produce smaller, more useful molecules. This process involves heating the hydrocarbons to vaporise them. The vapours are either passed over a hot catalyst or mixed with steam and heated to a very high temperature so that thermal decomposition reactions then occur.
The products of cracking include alkanes and unsaturated hydrocarbons called alkenes. Alkenes have the general formula C n
H
2n
.
Unsaturated hydrocarbon molecules can be represented in the following forms: C
3
Alkenes react with bromine water, turning it from orange to colourless.
H
6
, Displayed formula
The combustion of hydrocarbon fuels releases energy. During combustion the carbon and hydrogen in the fuels are oxidised.
Most fuels, including coal, contain carbon and/or hydrogen and may also contain some sulfur. The gases released into the atmosphere when a fuel burns may include carbon dioxide, water (vapour), carbon monoxide, sulfur dioxide and oxides of nitrogen. Solid particles (particulates) may also be released.
Sulfur dioxide and oxides of nitrogen cause acid rain, carbon dioxide causes global warming, and solid particles cause global dimming.
Sulfur can be removed from fuels before they are burned, for example in vehicles. Sulfur dioxide can be removed from the waste gases after combustion, for example in power stations
Lesson 1: Life is made of the same compounds as crude oil.
Key words: mixture, compounds, hydrocarbons, alkane.
Lesson 2: Crude oil is used to fuel aeroplanes.
Key words: Boiling point, vapourised, saturated, properties.
Lesson 3: Hydrocarbons all have similar properties as they are all made of the same things.
Key words: Cracking, unsaturated, catalyst.
Learning Intentions:
Students should develop an understanding that:
Crude oil is made of a mixture of many compounds, called hydrocarbons, which are made of hydrogen and carbon.
Hydrocarbons vary in chain length and can be named depending on the number of carbons in their chain.
Hydrocarbons structures can be given using displayed and structural formula.
Success Criteria:
Recall the elements that make up hydrocarbons.
Suggest names for hydrocarbons.
Model different hydrocarbon chain lengths using molymods and other modelling equipment.
Feedback Focus:
Knowledge input | Check | Development | REACH | Improvement
Details:
Peer assessment activity naming hydrocarbons.
Teacher verbal formative assessment and use of rubric to keep record of feedback in books.
Learning Intentions:
Students should develop an understanding that:
The many hydrocarbons which make up crude oil can be separated into fractions of similar chain length by fractional distillation.
Each of the fractions of crude oil has a specific use.
Success Criteria:
Identify the name of the process used to separate crude oil.
Explain how factional distillation works.
Research the uses of each chain length produced from fractional distillation.
Feedback Focus:
Knowledge input | Check | Development | REACH | Improvement
Details:
Self-assessed exam question using mark scheme.
Home learning: Fractional distillation 6 Mark exam questions
Learning Intentions:
Students should develop an understanding that:
Longer chain hydrocarbons can be broken down into smaller, more useful unsaturated hydrocarbons.
Alkene double bond can be tested for using bromine water.
Success Criteria:
Build models for the first 4 alkenes.
Explain the process of cracking.
Describe how to test for alkenes.
Feedback Focus:
Knowledge input | Check | Development | REACH | Improvement
Details:
Assess redraft of 6 mark question from lesson 2 using rubric
Teacher assessed of cracking equation questions
Year 10 Chemistry TRIPLE | Learning Cycle 2 | Medium Term Plan | Science 2015/16
Lesson 3: Hydrocarbons all have similar properties as they are all made of the same things.
Key words: Cracking, unsaturated, catalyst.
Learning Intentions:
Students should develop an understanding that:
Longer chain hydrocarbons can be broken down into smaller, more useful unsaturated hydrocarbons.
Alkene double bond can be tested for using bromine water.
Success Criteria:
Build models for the first 4 alkenes.
Explain the process of cracking.
Describe how to test for alkenes.
Feedback Focus:
Knowledge input | Check | Development | REACH | Improvement
Details:
Assess redraft of 6 mark question from lesson 2 using rubric
Teacher assessed of cracking equation questions
Lesson 4: Longer hydrocarbon chains release less energy than shorter chain hydrocarbons.
Key words: Combustion, particulates, incomplete, complete.
Learning Intentions:
Students should develop an understanding that:
Longer hydrocarbon chains release more energy when they are combusted.
Hydrocarbons produce water and carbon dioxide when combusted.
Success Criteria:
Recall products released by combustion of hydrocarbons.
Propose hydrocarbon chain length for unknown molecules.
Predict the properties of hydrocarbons based on their chain length.
Evaluate investigation.
Practical opportunity
Comparison of the energy content of different fuels, for example by heating a fixed volume of water
Feedback Focus:
Knowledge input | Check | Development | REACH | Improvement
Details:
Peer assessment of analysis of results using mark schemes and graphs of answers.
Lesson 5: The burning of fossil fuels is responsible for the damage to marine ecosystems.
Key words: Global warming, acid rain, global dimming.
Learning Intentions:
Students should develop an understanding that:
Combustion of hydrocarbons releases harmful products.
These products have different environmental effects.
Success Criteria:
Identify harmful products released by combustion of hydrocarbons.
Explain the effects harmful emissions have on the planet.
Research different methods used to limit harmful emissions.
Decide which environmental effect is most damaging and justify your answer.
Feedback Focus:
Knowledge input | Check | Development | REACH | Improvement
Details:
Self-assessment of investigation evaluation from previous lesson during starter.
Teacher assessed 6 mark exam question on implications of burning fossil fuels
Homework: Research the different types of biofuels and their economic, environmental and ethical implications.
Year 10 Chemistry TRIPLE | Learning Cycle 2 | Medium Term Plan | Science 2015/16
Line of enquiry two: Are polymers the most useful by-products of organic chemistry?
Intentions for learning from AQA GCSE specification:.
Alkenes can be used to make polymers such as poly(ethene) and poly(propene). In these reactions, many small molecules (monomers) join together to form very large molecules (polymers).
Polymers have many useful applications and new uses are being developed, for example: new packaging materials, waterproof coatings for fabrics, dental polymers, wound dressings, hydrogels, smart materials (including shape memory polymers).
Many polymers are not biodegradable, so they are not broken down by microbes and this can lead to problems with waste disposal.
Plastic bags are being made from polymers and cornstarch so that they break down more easily.
The properties of polymers depend on what they are made from and the conditions under which they are made. For example, low density (LD) and high density (HD) poly(ethene) are produced using different catalysts and reaction conditions.
Thermosoftening polymers consist of individual, tangled polymer chains. Thermosetting polymers consist of polymer chains with cross-links between them so that they do not melt when they are heated.
Biofuels, including biodiesel and ethanol, are produced from plant material. There are economic, ethical and environmental issues surrounding biofuels.
Ethanol can be produced by hydration of ethene with steam in the presence of a catalyst.
Ethanol can also be produced by fermentation with yeast, using renewable resources. This can be represented by: sugar
carbon dioxide + ethanol
Lesson 6: Car tyres are made from plants.
Lesson 7: Polymers form bulletproof crystals. Lesson 8: Cars can be run on corn.
Key words: Monomer, polymer, biodegradable.
Learning Intentions:
Students should develop an understanding that:
Alkene monomers are joined together to make large molecules called polymers.
Polymers have an extensive range of uses including plastic bags.
Most polymers are not biodegradable and cannot be broken down by microbes.
Success Criteria:
Propose names for polymers using alkene names.
Use structure and bonding to explain properties of polymers.
Write symbol equations for the polymerisation of ethene and propene.
Feedback Focus:
Knowledge input | Check | Development | REACH | Improvement
Details:
Redraft 6 marker based on teacher feedback
Self-assessed task naming polymers and writing equations,
Teacher verbal formative feedback with use of models.
Key words: High density, low density, hydrogen bonds,
thermosoftening.
Learning Intentions:
Students should develop an understanding that:
The properties of polymers can be changed depending on the type of catalyst and reaction conditions.
Success Criteria:
Compare and contrast the different properties of polymers when they are made under different conditions.
Construct models for high density and low density polymers.
Evaluate the social, economic and environmental impact of the uses, disposal and recycling of polymers.
Practical opportunity:
Making slime polymers – borax needed and PVA glue.
Food colouring.
Feedback Focus:
Knowledge input | Check | Development | REACH | Improvement
Details:
Peer assessment using rubric for exam question evaluating environmental impacts. Redrafted exam question to be teacher assessed.
Key words: biofuels, hydration, catalyst, fermentation.
Learning Intentions:
Students should develop an understanding that:
A range of alternative fuel resources are available using natural biological resources.
Success Criteria:
Recall the structural formula for ethanol.
Describe the two methods for the production of ethanol.
Evaluate which biofuels are best considering economic, ethical and environmental issues.
Feedback Focus:
Knowledge input | Check | Development | REACH | Improvement
Details:
Teacher assessment of home learning task through mini-test
Peer assessment using mark scheme of exam question.