Chapter 8: Major Elements
advertisement
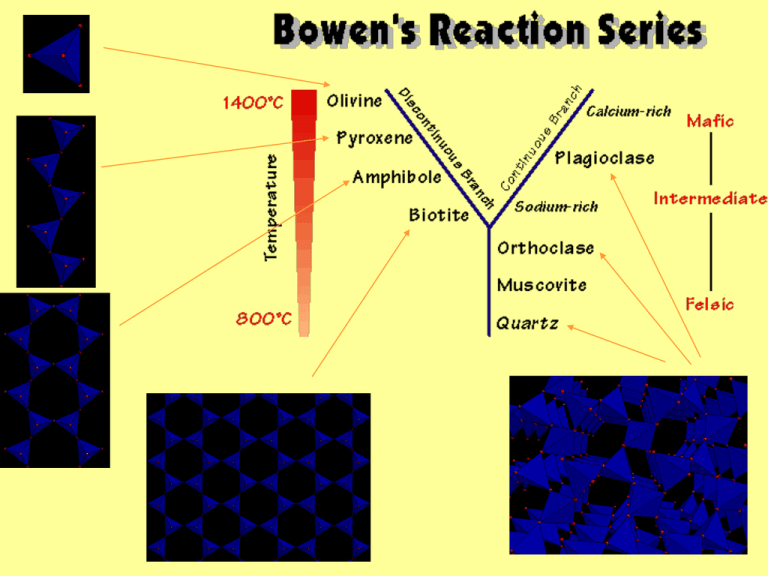
NASA News 03-15-06 Scientists say the minerals found in Stardust aerogels include magnesium olivine (forsterite) "In the coldest part of the solar system, we have found samples that have formed at extremely high temperatures. So, the hottest samples in the coldest place." Tectosilicates Feldspars Substitute Al3+ for Si4+ allows Na+ or K+ to be added Albite-Orthoclase Substitute two Al3+ for Si4+ allows Ca2+ to be added Albite-Anorthite Albite: NaAlSi3O8 Melt-crystal equilibrium 1 When crystal comes out of melt, some ions go in easier more Ca rich crystals form 1st Precipitated crystals react with cooling liquid, eventually will reequilibrate back, totallly cooled magma xstals show same composition Magma at composition X (30% Ca, 70% Na) cools first xstal bytownite X Melt-crystal equilibrium 1 Magma at composition X (30% Ca, 70% Na) cools first crystal bytownite (73% Ca, 27% Na) This shifts the composition of the remaining melt such that it is more Na-rich (Y) What would be the next crystal to precipitate? Finally, the last bit would crystallize from Z X Y Z Melt-crystal equilibrium 1b Precipitated crystals react with cooling liquid, eventually will reequilibrate back, totally cooled magma xstals show same composition UNLESS it cools so quickly the xstal becomes zoned or the early precipitates are segregated and removed from contact with the bulk of the melt Why aren’t all feldspars zoned? Kinetics, segregation IF there is sufficient time, the crystals will reequilibrate with the magma they are in – and reflect the total Na-Ca content of the magma IF not, then different minerals of different composition will be present in zoned plagioclase or segregated from each other physically Melt-crystal equilibrium 2 - miscibility 2 component mixing and separation chicken soup analogy, cools and separates Fat and liquid can crystallize separately if cooled slowly Miscibility Gap – no single phase is stable SOUP of X composition cooled in fridge Y vs freezer Z 100 SOUP Temperature (ºC) X 50 Y 0 fats ice Z -20 10 Miscibility Gap 30 50 Water % fat in soup 70 90 Fat Melt-crystal equilibrium 2 - miscibility 2 component mixing and separation chicken soup analogy, cools and separates Fat and liquid can crystallize separately if cooled slowly Miscibility Gap – no single mineral is stable in a composition range for x temperature monalbite anorthoclase 1100 Temperature (ºC) high albite 900 700 500 sanidine intermediate albite orthoclase low albite microcline Miscibility Gap 300 10 Orthoclase KAlSi3O8 30 50 % NaAlSi3O8 70 90 Albite NaAlSi3O8








