Trial Overview - Clinical Trial Results
advertisement

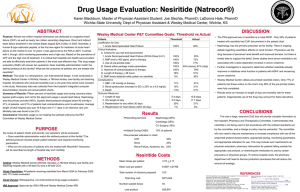
THE NAPA TRIAL: Nesiritide Administered Peri-Anesthesia in Patients Undergoing Cardiac Surgery Mark J. Russo, MD, MS Division of Cardiothoracic Surgery & International Center for Health Outcomes and Innovation Research College of Physicians and Surgeons, Columbia University, New York, NY BACKGROUND • Nesiritide is recombinant human B-type natriuretic peptide Introduction Methods Results Summary • When administered to patients with heart failure, it: – decreases preload and afterload – decreases pulmonary vascular resistance – increases cardiac output • In some studies: – increased urine output – reduced diuretic requirements – suppression of aldosterone, endothelin, norepinephrine BACKGROUND Introduction Methods Results Summary • Nesiritide is approved for treatment of patients with acutely decompensated congestive heart failure who have dyspnea at rest or with minimal activity • Several small, retrospective studies suggested beneficial effects in patients undergoing cardiac surgery OBJECTIVES Introduction Methods Results Summary To explore the effects of perioperative administration of nesiritide on clinical outcomes and safety in heart failure patients undergoing cardiac surgery. NAPA TRIAL DESIGN Introduction Methods Results Summary • Multi-center (54 centers) • Randomized • Double-blind • Placebo-controlled NAPA TRIAL DESIGN Introduction Methods Results Summary • LV dysfunction (EF≤40%) • NYHA Class II - IV • undergoing CABG ± MVS • using cardiopulmonary bypass EXCLUSION CRITERIA Introduction Methods Results Summary • Planned AVR/r • Off-pump • Ongoing or chronic dialysis • Hemodynamic criteria – Mean PAP < 15 mm Hg – CVP < 6 mm Hg – SBP < 90 mm Hg STUDY PROTOCOL Introduction Methods Results Summary OUTCOME MEASURES Introduction Methods Results Summary • Mean peak change in serum Cr and GFR through hospital discharge or POD #14 • Cardiac, renal, and pulmonary adverse events • Mortality (30-day and 180-day) • Mean ICU LOS & total hospital LOS STUDY POPULATION Male Sex (%) Introduction Methods Results Summary Age (yrs) Ejection Fraction (%) Serum Creatinine (mg/dL) GFR (mL/min/1.73 m2) Baseline BNP (pg/mL) SBP (mm Hg) Mean PAP (mm Hg) Nesiritide (n=141) Placebo (n=138) 79% 78% 63.6 ± 10.5 64.1 ± 11.3 29.7 ± 7.5 30.1 ± 7.3 1.07 ± 0.4 82.0 ± 30.3 431 ± 615 122 ± 21 25.4 ± 8.8 1.11 ± 0.4 77.6 ± 28.1 406 ± 511 120 ± 21 25.6 ± 8.7 STUDY POPULATION Medic al His t ory n (% ) Nes irit ide P lac ebo (n= 141) (n= 138) Non– ins ulin-dependent diabet es mellit us 43 (31%) 45 (32%) Introduction Methods Ins ulin-dependent diabet es mellit us 26 (19%) 23 (16%) Chronic obs t ruc t iv e pulmonary dis eas e 25 (18%) 25 (18%) Results Summary Ot her pulmonary dis eas e 17 (12%) 21 (15%) P eripheral v as c ular dis eas e 30 (22%) 29 (21%) 8 (6%) 6 (4%) 33 (25%) 28 (21%) Diabet ic nephropat hy Ot her c hronic renal dis eas e 30-DAY ADVERSE EVENTS* Introduction Methods Results Summary MEAN PEAK CHANGE IN SCr* Introduction Methods Results Summary *Through hospital discharge or study Day 14, whichever came first RENAL BENEFIT WAS GREATER IN PATIENTS WITH RENAL DYSFUNCTION AT BASELINE Baseline SCr ≤ 1.2mg/dl Introduction Methods Results Summary Baseline SCr > 1.2mg/dl 180-DAY SURVIVAL WAS IMPROVED WITH NESIRITIDE Introduction Methods Results Summary LENGTH OF STAY WAS SHORTER WITH NESIRITIDE Introduction Methods Results Summary LIMITATIONS Introduction Methods Results Summary • Usual-care medications and other treatment interventions were not specified in the protocol. • Patients enrolled in this study represent only a subset of patients undergoing CABG • The 180-day mortality end point was added late in the study as an additional safety end point NAPA FINDINGS Introduction Methods • Improved Survival at 180 days • Improved Postop Renal Function – Greater improvement in patients with renal dysfunction at baseline Results Summary • Decreased LOS Safety and Efficacy of Therapies for Acute Decompensated Heart Failure Clyde W. Yancy, MD Medical Director Baylor Heart and Vascular Institute Baylor University Medical Center Dallas, TX Disclosure Information Clyde W. Yancy, MD • Grants/Research Support: GlaxoSmithKline; Medtronic, Inc.; NitroMed, Inc.; Scios Inc. • Support/Consultant: AstraZeneca Pharmaceuticals LP; GlaxoSmithKline; Medtronic, Inc.; NitroMed, Inc.; Scios Inc. • Speaker’s Bureau: GlaxoSmithKline; Novartis Pharmaceuticals Corporation Outcomes in Patients Hospitalized With HF 100 Hospital Readmissions 100 Mortality 75 75 50% 50 50% 50 33% 20% 25 25 0 0 30 days 6 mo Median hospital LOS: 6 days Jong P et al. Arch Intern Med. 2002;162:1689 12% 30 days 12 mo 5 yr Annual mortality rateNYHA class III HF12% [COPERNICUS DATA] NYHA class II HF7% [SCD-HeFT DATA] Explanations for Increased Mortality Risk in ADHF • Absence of understanding - What is the relevant pathophysiology of ADHF? • Acts of commission - Administration of agents that cause harm • Acts of omission - Failure to administer therapies known to be effective • Failure of follow-up OR (95% CI) of characteristics as predictors of short-term and long-term all-cause mortality after hospitalization with acute HF Characteristic Age •55-64 •>85 BMI >30 Edema Serum urea nitrogen (per mg/dL rise) COPD Hypertension Stroke Heart failure Peripheral vascular disease 3 mo 5y 0.30 (0.13–0.70) 1.55 (0.88–2.74) 0.55 (0.40–0.77) 1.20 (0.92–1.56) 1.02 (1.01–1.03) 1.27 (0.80–2.01) 6.27 (3.94–10.00) 0.62 (0.47–0.82) 1.38 (1.07–1.77) 1.02 (1.01–1.03) 1.19 (0.94–1.52) 0.77 (0.61–0.99) 1.40 (1.04–1.90) 1.04 (0.77–1.40) 0.76 (0.55–1.06) 1.97 (1.53–2.54) 1.00 (0.79–1.28) 1.49 (1.04–2.13) 2.20 (1.72–2.83) 1.42 (1.02–1.97) Goldberg RJ et al. Arch Intern Med 2007; 167:490496. Treatment Options for Acute HFTODAY- are these agents safe and Diuretics, effective? Aquaretics & Ultrafiltration Fluid volume Vasodilators Preload and/or Afterload Inotropes Natriuretic Peptides Contrac -tility Fluid volume Preload Afterload Neurohormones Increase lusitropy Potential Deleterious Effects of Diuretics and Cardiorenal Syndrome of HF Increased morbidity and mortality Pathologic remodeling Congestion Neurohormonal activation Diuretic resistance Impaired renal function Diuretic therapy Neurohormonal activation Vasoconstriction Diminished blood flow Decreased renal perfusion Diuretic Resistance Predicts Mortality in Advanced HF Neuberg GW et al. Am Heart J. 2002;144:31. Treatment Options for Acute HFTODAY- are these agents safe and Diuretics, effective? Aquaretics & Ultrafiltration Fluid volume Vasodilators Preload and/or afterload Inotropes Natriuretic Peptides Contrac -tility Fluid volume Preload Afterload Neurohormones Increase lusitropy Vasodilators Nitroglycerin Nitroprusside Nesiritide • Reduces preload • Relieves ischemia • Improves symptomatic HF • Reduces afterload • Reduces blood pressure • Increases cardiac output • Reduces preload & afterload • Increases cardiac output • Decreases neurohormonal activation • Relieves dyspnea None of the above have been shown to improve mortality for ADHF in randomized controlled clinical trials Hemodynamic Effects of Nesiritide vs Placebo vs IV NTG Time on Study Drug (hr) Change From Baseline in PCWP (mm Hg) 0 0.25 0.5 1 2 3 6 9 12 24 36 48 0 PCWP – Placebo –1 PCWP – IV NTG –2 PCWP – Nesiritide –3 –4 –5 –6 During 3-hr placebo period Placebo n = 62 IV NTG n = 60 Nesiritide n = 124 * † * † * –7 –8 † * * † * † † –9 † † After 3-hr period IV NTG n = 92 Nesiritide n = 154 End of Placebo-Controlled Period *P0.05 vs placebo †P0.05 vs IV NTG Publication Committee for the VMAC Investigators. JAMA. 2002;287:1531 ns 5 Mins *Added VMAC: Dyspnea Improvement Proportion of Subjects (%) Dyspnea at 3 hr 100 90 80 70 60 50 40 30 20 10 0 10 20 30 40 # P=0.034 P=0.191 Nitroglycerin* (n = 143) 1 Hour to standard care1 Hour ® or nitroglycerin ® or nitroglycerin orMins compared to placebo Natrecor compared to for placebo 5 1 Hour Publication Committee the VMAC red to nitroglycerin ompared to nitroglycerin 5 Mins® 1 Hour # # # # Nesiritide* Placebo* (n = 204) (n = 142) 33Hours Hours Hours Investigators. JAMA.3 2002;287:1531 3 Hours Markedly Better Markedly Better Markedly Markedly Moderately Better Better Better Moderately Markedly Markedly better Better Moderately Moderately Better Minimally Moderately better Better Minimally Better Moderately Better Minimally Minimally better Minimally Better MinimallyBetter MinimallyMinimally Markedly No Change Markedly No change Change Better No Worse MinimallyWorse MinimallyMarkedly No Change MinimallyMarkedly No Change MinimallyWorse Minimally MinimallyMarkedly No Change Worse Markedly No Markedly No Change Change markedly worse Worse MinimallyWorse Worse MinimallyMarkedly No Change Markedly Worse No Change MinimallyWorse Markedly No Change Worse What are the risks of nesiritide therapy? Risk of Worsening Renal Failure: Nesiritide Relative to Control Therapies Sackner-Bernstein JD et al. Circulation 2005;111:1487-1491. ≤ 0.03 mcg/kg/min ≤ 0.015 mcg/kg/min ≤ 0.06 mcg/kg/min P ≤ 0.003 P ≤ 0.012 P ≤ 0.002 Odds Ratios Of Worsening Serum Creatinine (>0.5 mg/dL) By Nesiritide Dose Group Nesiritide Worse Nesiritide Better 0.01 mcg/kg/min P=0.17 0.015 mcg/kg/min P=0.02 0.03 mcg/kg/min P=0.001 0 1 2 3 4 Odds Ratio (and 95% confidence intervals) Abraham WT. Serum Creatinine Elevations in Patients Receiving Nesiritide are Related to Starting Dose HFSA 2005 5 DID WE LEARN ANYTHING FROM FUSION-II? FUSION II: Primary Composite Endpoint Through Week 12 Placebo Nesiritide Combined Combined N=306 N=605 *P-value All cause mortality and CV/renal hospitalization† 36.8% 36.7% 0.79 All Cause Mortality 9.6% 9.5% 0.98 CV/renal hospitalization 33.9% 32.9% 0.95 *P value: NES vs. placebo stratified by dose group †Modified ITT: all treated ITT patients SAFETY Protocol Specified Changes in Serum Creatinine* P=0.046 Nesiritide Combined 39% Placebo Combined 32% P=0.931 16% P=0.458 4% >0.5 mg/dL 5% >100% >=50% to at least 2 mg/dL Serum Creatinine Increase from Baseline *Outpatient Clinic Visit Values Only 15% Treatment Options for Acute HFTODAY- are these agents safe and Diuretics, effective? Aquaretics & Ultrafiltration Fluid volume Vasodilators Preload and/or afterload Inotropes Natriuretic Peptides Contractility Fluid volume Preload Afterload Neurohormones Increase lusitropy Calcium Sensitizing Agents: Overview • Increase cardiac contractility by increasing sensitivity of myofilaments to Ca2+ • Do not increase intracellular Ca2+ levels • Generate increased contractile force for a given level of intracellular Ca2+ • May provide a “more economical” increase in inotropic effect (i.e. without a significant increase in myocardial O2 consumption) Mathew and Katz, Drugs Aging, 1998 Haikala and Linden, J Cardiovasc Pharmacol, 1995 Relationship between i[Ca2+] and Cell Shortening % cell shortening 15 Ca2+ sensitizers Desensitizing agents 10 5 0 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 Intracellular calcium concentration Hemodynamic Effects and Mortality Rates of Levosimedan vs. Dobutamine-LIDO End point Levosimedan Dobutamine HR (95% CI) p value Hemodynamic improvement 28% 15% 1.9 (1.1-3.3) 0.022 Mortality at 180 days 26% 38% 0.57 (0.340.95) 0.029 Follath et al. Lancet 2002;360:196 REVIVE-2 • 600 pts c ADHF • Randomized to placebo vs. levosimendan • Composite endpoint- Improvement in 6 hrs - Requirement for vasoactive Rx - Death 70 60 50 40 Improved no change Worsened 30 20 10 0 clinical status 75% of patients treated with Levosimendan were either unchanged or worsened Approximate Time-dependent Rates of “Moderate or Marked" Improvement in Patient Global Assessment Levosimendan, Interval n=299 (%) 24 h* 60 48 h 63 5d 76 *infusions halted at 24 hours Placebo, n=301 (%) 46 58 65 Packer M et al. American Heart Association Scientific Sessions 2005; November 13–16, 2005; Dallas, TX. p 0.026 0.053 0.001 Adverse Events in REVIVE-2 Selected adverse events Levosimendan (%) Placebo (%) Hypotension 49.2 35.5 Headache 29.4 14.6 Ventricular tachycardia 24.1 16.9 Cardiac failure 22.4 26.6 Atrial fibrillation 8.4 0.2 Ventricular extrasystoles 7.4 0.2 Packer M et al. American Heart Association Scientific Sessions 2005; November 13–16, 2005; Dallas, TX. All-cause Mortality by Time since the First Infusion in the SURVIVE-W Trial Interval Analysis Levosimendan, Dobutamine, HR n=664 (%) n=663 (%) (95% CI) 180 d Primary end point 26 28 0.91 (0.74-1.13) 31 d Secondary 12 end point 14 0.85 (0.63-1.15) 5d Post hoc 6.0 0.72 (0.44-1.16) 4 Mebazaa A. American Heart Association Scientific Sessions 2005; November 13–16, 2005; Dallas, TX. Evaluation and Management of Patients With ADHF: Recommendations • Patients admitted with ADHF and evidence of fluid overload be treated initially with loop diuretics • When congestion fails to improve in response to diuretic therapy, the following options should be considered - Sodium and fluid restriction - Increased doses of loop diuretics - Continuous infusion of a loop diuretic - Addition of a second type of diuretic - Ultrafiltration • In the absence of symptomatic hypotension, IV NTG, NTP, or nesiritide may be considered as an addition to diuretic therapy for rapid improvement of congestive symptoms in patients with ADHF Adams KF et al. J Card Fail. 2006;12:10 Section 12: Evaluation and Management of Patients with ADHF • 12.15- “In the absence of hypotension, IV NTG, sodium nitroprusside or nesiritide may be considered as an addition to diuretic therapy for rapid improvement of congestive symptoms in patients admitted with ADHF”. [Strength of evidence B] • 12.17- “Intravenous vasodilators, (nitroprusside, nitroglycerin or nesiritide) may be considered in patients with ADHF and advanced HF who have persistent severe HF despite aggressive treatment with diuretics and standard oral therapies” [Strength of evidence C] J Cardiac Failure. 2006;12:10–38 Evaluation and Management of Patients With ADHF: Recommendations • 12.16 IV vasodilators (IV NTG or NTP) and diuretics are recommended for rapid relief in patients with acute pulmonary edema or severe hypertension • IV inotropes (milrinone or dobutamine) may be considered to relieve symptoms and improve end-organ function in patients with advanced HF J Cardiac Failure. 2006;12:10–38 Question & Answer Thank You! Please make sure to hand in your evaluation and pick up a ClinicalTrialResults.org flash drive