Cryogenic Separations - Chemical Engineering
Authors: Jared Watkins, Juan Tellez
INTRODUCTION
Key steps of the cryogenic air distillation process including air compression, air cooling and purification, heat exchange, refrigeration, internal product compression and rectification were investigated. The study of this process has become increasingly popular in accordance with the rise in demand for these components within industry such as mining and semiconductors. Within the separation unit the air is separated via distillation at very low temperatures to take advantage of the boiling point differences of the air components in a process known as rectification. The distillation column can be designed depending on the specific products needed, with the most common design being the double column system with an adjacent argon unit. The energy needed for the very low temperatures constitutes most of cost of production and so it is of interest to optimize the process and obtain maximum efficiency. While involving a high initial capital cost air separation units have relatively high yields and can obtain large volumes of high purity gases or liquids.
EQUATIONS
1. Equilibrium Condition
2. Mass Balance 𝑦 𝑖 𝑛 𝑓
= 𝑘 𝑖 𝑥 𝑖 𝑥 𝑓
= 𝑛
𝐷 𝑥
𝐷
+ 𝑛
𝐵 𝑥
𝐵 𝑛 𝑓
= 𝑛
𝐷
+ 𝑛 𝑥 𝑓
𝐵
−𝑥
𝐵 𝑛
𝐷
= 𝑛 𝑓 𝑥
𝐷
−𝑥
𝐵
3.
Energy Balance 𝑑𝐸 𝑑𝑡
= 𝑛
6. Peng –Robinson EOS
𝑉 𝑖𝑛 ℎ
𝑉 𝑖𝑛
+ 𝑛
𝐿 𝑖𝑛
4. Rectifying Section Operating Line
5. Stripping Section Operating Line ℎ
𝐿 𝑖𝑛
− 𝑛
𝑉 𝑜𝑢𝑡
𝑅 𝑦 =
𝑅+1
𝑅 ℎ
𝑉 𝑜𝑢𝑡 𝑥 + 𝑦 = 𝑥 + 𝑥
𝐷
𝑅+1 𝑥
𝐵
𝑅+1
𝑅𝑇
+ 𝑛
𝐿 𝑜𝑢𝑡
𝑅+1 𝑎 𝑇
𝑃 = −
𝑉−𝑏 ℎ
𝑉 𝑉+𝑏 +𝑏 𝑉−𝑏
𝐿 𝑜𝑢𝑡
7. Joule-Thompson Effect
Where 𝑦 𝑖
µ
𝐽𝑇
=
𝜕𝑉
𝜕𝑇 𝐻
=
𝑇
=vapor phase composition of component i, and 𝑥 𝑖
𝜕𝑉
𝜕𝑇 𝑃
𝐶
𝑃
−𝑉 the liquid phase, n=molar flow rate, h=enthalpy, and R=reflux ratio
Figure 7: Column Tray schematic.
Retrieved from Roffel, 2000. Liquid oxygen flows down and becomes more concentrated as nitrogen vapor rises. Modern plants use structured packing's to offer maximum surface area and a low pressure drop.
Table 1: Air composition and thermodynamic properties of its components at 101.1 Pa. Retrieved from Agrawal, 2000 .
Temperature (K)
Figure 1: Vapor Pressure curve for atmospheric gasses, showing the dew point and bubble point of air and its major components. Retrieved from Linde Engineering.
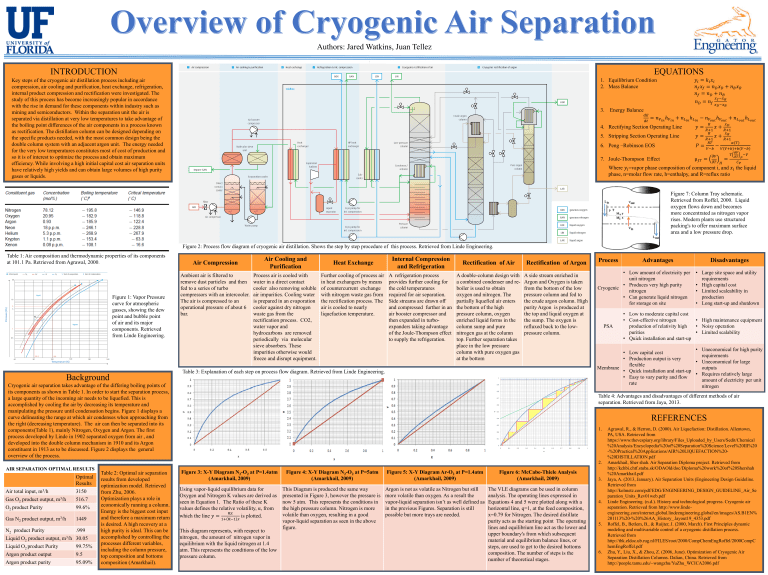
Figure 2: Process flow diagram of cryogenic air distillation. Shows the step by step procedure of this process. Retrieved from Linde Engineering.
Air Compression
Air Cooling and
Purification
Heat Exchange
Internal Compression and Refrigeration
Rectification of Air Rectification of Argon
Ambient air is filtered to remove dust particles and then fed to a series of turbo compressors with an intercooler.
The air is compressed to an operational pressure of about 6 bar.
Process air is cooled with water in a direct contact cooler also removing soluble air impurities. Cooling water is prepared in an evaporation cooler against dry nitrogen waste gas from the rectification process. CO2, water vapor and hydrocarbons are removed periodically via molecular sieve absorbers. These impurities otherwise would freeze and disrupt equipment.
Further cooling of process air in heat exchangers by means of countercurrent exchange with nitrogen waste gas from the rectification process. The air is cooled to nearly liquefaction temperature.
A refrigeration process provides further cooling for the cold temperatures required for air separation.
Side streams are drawn off and compressed further in an air booster compressor and then expanded in turboexpanders taking advantage of the Joule-Thompson effect to supply the refrigeration.
A double-column design with a combined condenser and reboiler is used to obtain oxygen and nitrogen. The partially liquefied air enters the bottom of the high pressure column, oxygen enriched liquid forms in the column sump and pure nitrogen gas at the column top. Further separation takes place in the low pressure column with pure oxygen gas at the bottom
A side stream enriched in
Argon and Oxygen is taken from the bottom of the low pressure column and fed to the crude argon column. High purity Argon is produced at the top and liquid oxygen at the sump. The oxygen is refluxed back to the lowpressure column.
Table 3: Explanation of each step on process flow diagram. Retrieved from Linde Engineering.
Process Advantages Disadvantages
Cryogenic
•
Low amount of electricity per unit nitrogen
• Produces very high purity nitrogen
• Can generate liquid nitrogen for storage on site
•
Large site space and utility requirements
• High capital cost
•
Limited scaleability in production
•
Long start-up and shutdown
PSA
• Low to moderate capital cost
•
Cost-effective nitrogen production of relativity high purities
•
Quick installation and start-up
•
High maintenance equipment
• Noisy operation
•
Limited scalability
Membrane
• Low capital cost
•
Production output is very flexible
• Quick installation and start-up
•
Easy to vary purity and flow rate
•
Uneconomical for high purity requirements
• Uneconomical for large outputs
•
Requires relatively large amount of electricity per unit nitrogen
Table 4: Advantages and disadvantages of different methods of air separation. Retrieved from Jaya, 2013.
Background
Cryogenic air separation takes advantage of the differing boiling points of its components as shown in Table 1. In order to start the separation process, a large quantity of the incoming air needs to be liquefied. This is accomplished by cooling the air by decreasing its temperature and manipulating the pressure until condensation begins. Figure 1 displays a curve delineating the range at which air condenses when approaching from the right (decreasing temperature). The air can then be separated into its components(Table 1), mainly Nitrogen, Oxygen and Argon. The first process developed by Linde in 1902 separated oxygen from air , and developed into the double column mechanism in 1910 and its Argon constituent in 1913 as to be discussed. Figure 2 displays the general overview of the process.
AIR SEPARATION OPTIMAL RESULTS
Optimal
Results
Air total input, m 3 /h 3150
Gas O
2 product output, m 3 /h 516.7
O
2 product Purity 99.6%
Gas N
2 product output, m 3 /h 1449
N
2 product Purity .999
Liquid O
2 product output, m 3 /h 30.05
Liquid O
2 product Purity
Argon product output
99.75%
9.5
Argon product purity 95.09%
Table 2: Optimal air separation results from developed optimization model. Retrieved from Zhu, 2006.
Optimization plays a role in economically running a column.
Energy is the biggest cost input and therefore a maximum return is desired. A high recovery at a high purity is ideal. This can be accomplished by controlling the processes different variables, including the column pressure, top composition and bottoms composition (Amarkhail).
Figure 3: X-Y Diagram N
2
-O
2
(Amarkhail, 2009) at P=1.4atm Figure 4: X-Y Diagram N
2
-O
2
(Amarkhail, 2009) at P=5atm Figure 5: X-Y Diagram Ar-O
2
(Amarkhail, 2009) at P=1.4atm Figure 6: McCabe-Thiele Analysis
(Amarkhail, 2009)
Using vapor-liquid equilibrium data for
Oxygen and Nitrogen K values are derived as seen in Equation 1. The Ratio of these K values defines the relative volatility, α, from
α 𝑥 which the line 𝑦 =
1+
α
−1 𝑥 is plotted.
This diagram represents, with respect to nitrogen, the amount of nitrogen vapor in equilibrium with the liquid nitrogen at 1.4 atm. This represents the conditions of the low pressure column.
This Diagram is produced the same way presented in Figure 3, however the pressure is now 5 atm. This represents the conditions in the high pressure column. Nitrogen is more volatile than oxygen, resulting in a good vapor-liquid separation as seen in the above figure.
Argon is not as volatile as Nitrogen but still more volatile than oxygen. As a result the vapor-liquid separation isn’t as well defined as in the previous Figures. Separation is still possible but more trays are needed.
The VLE diagrams can be used in column analysis. The operating lines expressed in
Equations 4 and 5 were plotted along with a horizontal line, q=1, at the feed composition, x=0.79 for Nitrogen. The desired distillate purity acts as the starting point The operating lines and equilibrium line act as the lower and upper boundary's from which subsequent material and equilibrium balance lines, or steps, are used to get to the desired bottoms composition. The number of steps is the number of theoretical stages.
REFERENCES
1.
Agrawal, R., & Herron, D. (2000). Air Liquefaction: Distillation. Allentown,
PA, USA. Retrieved from https://www.thevespiary.org/library/Files_Uploaded_by_Users/Sedit/Chemical
%20Analysis/Encyclopedia%20of%20Separation%20Science/Level%20III%20
-%20Practical%20Applications/AIR%20LIQUEFACTION%20-
%20DISTILLATION.pdf
2.
Amarkhail, Sher shah. Air Separation Diploma project. Retrieved from http://kchbi.chtf.stuba.sk/ODAOld/doc/Diploma%20work%20of%20Shershah
%20Amarkhail.pdf
3.
Jaya, A. (2013, January). Air Separation Units (Engineering Design Guideline.
Retrieved from http://kolmetz.com/pdf/EDG/ENGINEERING_DESIGN_GUIDELINE_Air_Se paration_Units_Rev01web.pdf
4.
Linde Engineering. (n.d.). History and technological progress. Cryogenic air separation. Retrieved from http://www.lindeengineering.com/internet.global.lindeengineering.global/en/images/AS.B1EN%
201113%20-%20%26AA_History_.layout19_4353.pdf
5.
Roffel, B., Betlem, B., & Ruijter, J. (2000, March). First Principles dynamic modeling and multivariable control of a cryogenic distillation process.
Retrieved from http://tbk.eldoc.ub.rug.nl/FILES/root/2000/CompChemEngRoffel/2000CompC hemEngRoffel.pdf
6.
Zhu, Y., Liu, X., & Zhou, Z. (2006, June). Optimization of Cryogenic Air
Separation Distillation Columns. Dalian, China. Retrieved from http://people.tamu.edu/~wangzhu/YuZhu_WCICA2006.pdf







