Unit 3 covalent compounds - jamietucker13
advertisement

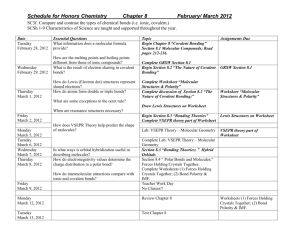
Unit 3: Covalent Bonding Lewis Structures, VSEPR, and intermolecular forces Dates: 10/27/14 – 11/13/14 Goals: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Be able to define the vocabulary listed below. Be able to list the diatomic molecules (there are 7). Be able to draw electron dot diagrams and lewis structures. Be able to predict single, double and triple bonds and how many electrons are shared in each. Be able to identify resonance structures. Be able to predict molecular shapes of compounds (VSEPR). Be able to represent polarity in a structural formula. Describe how mixtures can be separated based on polarity of substances. Be able to calculate and Rf value in a chromatography lab. Be able to calculate average atomic mass. Book/Notes 9.3 Molecular structures pp 252-258 9.2 Naming molecules pp 248 – 251 9.4 Molecular shape pp 259 – 262 9.5 Electronegativity and Polarity pp 263 - 267 Vocabulary: resonance Electron dot diagram Chromatography, Linear Rf value Trigonal Octahedral bipyramidal Single bond Double bond Labs/Projects: Splatter test Chromatography Halloweenium Lewis structure Hybridization VSEPR model Trigonal planar Tetrahedral Covalent bond Molecule Trigonal pyramidal Sigma bond Triple bond Polar covalent Nonpolar covalent Structural formula Bent Pi bond Covalent network solid Day 1 2 3 4 5 6 7 8 9 10 11 12 13 14 M: 10/27 T: 10/28 W: 10/29 Th: 10/30 F: 10/31 M: 11/3 T: 11/4 W: 11/5 Th: 11/6 F: 11/7 M: 11/10 T: 11/11 W: 11/12 Th:11/13 Go over test, make test corrections. Covalent naming. Finish covalent naming. Electron dot notation and lewis structures. Lewis structures practice. Naming Quiz. Chromatography lab. Begin lab by reading the background and discussing procedure. Crush and pulverize leaves. Halloweenium and Chromatography . Calculate the average atomic mass of the element “Hw” and identify pigments of fall leaves based upon Rf values. VSEPR introduction. Splatter test lab. Worksheet reinforcing intermolecular forces. VSEPR practice and molecular geometry VSEPR practice, molecular geometry, and vocabulary practice. No School. PT conferences all day. Dimensional Analysis Activity. No School. Veteran’s day Review stations Test! Homework calendar website: https://sites.google.com/a/k12.sd.us/jamietucker/Chemistry/covalent-bonding Quizlet website: http://quizlet.com/45686784/covalent-bonding-flash-cards/ and http://quizlet.com/45686684/covalent-naming-prefixes-flash-cards/ Wiki-Review Website http://jamietucker13.wikispaces.com/Chemistry+L Standards: Indicator 1: Describe structures and properties of, and changes in, matter. 9-12.P. 1.2. Students are able to describe ways that atoms combine. 9-12.P. 1.2A Students are able to predict electron configuration, ion formation, reactivity, compound formation, periodic trends, and types of compounds formed based on the location on the Periodic Table. 9-12.P.1.6A. Students are able to perform stoichiometric calculations. 9-12.P.1.8A. Students are able to use models to make predictions about molecular structure, chemical bonds, chemical reactivity, and polarity of molecules. Indicator 2: Apply the skills necessary to conduct scientific investigations. 9-12.N.2.1. Students are able to apply science process skills to design and conduct student investigations. 9-12.N.2.2. Students are able to practice safe and effective laboratory techniques.