Arboviruses
advertisement

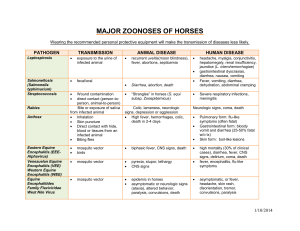
Chair of Medical Biology, Microbiology, Virology, and Immunology Lecturer As. Prof. O.Pokryshko The term ARBO is an abbreviation of "ARthropod BOrne". "Arbovirus" is the name given to Arthropodborne viruses, that is, viruses that are transmitted to vertebrates, such as people and mammals, by blood-feeding insects called arthropods. Vertebrate infection occurs when the infected insect bites an animal or person and takes a blood meal. . They can multiply in the tissues of the arthropod without evidence of disease or damage. The vector acquires a lifelong infection through the ingestion of blood from a viremic vertebrate. All arboviruses have an RNA genome, and most have a lipid-containing envelope and consequently are inactivated by ether or sodium deoxycholate. Current taxonomic status of some arboviruses Togaviridae Flaviviridae Bunyaviridae Reoviridae Rhabdoviridae Arenaviridae Nodaviridae Genus Alphavirus Genus Flavivirus Genus Bunyavirus Genus Orbivirus Genus Vesiculovirus Genus Arenavirus Approximately 80 arboviruses known to cause human disease Transmission Cycles Man - arthropod - man e.g. dengue, urban yellow fever. Reservoir may be in either man or arthropod vector. In the latter transovarial transmission may take place. Animal - arthropod vector - man e.g. Japanese encephalitis, EEE, WEE, jungle yellow fever. The reservoir is in an animal. The virus is maintained in nature in a transmission cycle involving the arthropod vector and animal. Man becomes infected incidentally. Both cycles may be seen with some arboviruses such as yellow fever. Animal Reservoirs In many cases, the actual reservoir is not known. The following animals are implicated as reservoirs Birds Japanese encephalitis, St Louis encephalitis, EEE, WEE Pigs Japanese encephalitis Monkeys Yellow Fever Rodents VEE, Russian Spring-Summer encephalitis Examples of Arthropod Vectors Aedes Aegyti Culex Mosquito Assorted Ticks Phlebotmine Sandfly Diseases Caused Fever and rash - this is usually a non-specific illness resembling a number of other viral illnesses such as influenza, rubella, and enterovirus infections. The patients may go on to develop encephalitis or haemorrhagic fever. Encephalitis - e.g. EEE, WEE, encephalitis, Japanese encephalitis. Haemorrhagic fever - e.g. yellow fever, dengue, Crimean-Congo haemorrhagic fever. St Louis Structures of Alphaviruses Principal medically important alphaviruses Virus Antigenic Clinical Syndrome Vector Host Distribution Eastern equine encephalitis Encephalitis (EEE) Mosquito Birds Americas Western equine encephalitis Encephalitis (WEE) Mosquito Birds North America Venezuelan equine encephalitis Febrile illness, encephalitis (VEE) Mosquito Rodents, horses Americas Virus Antigenic Clinical Syndrome Chikunguny (CHIK) Vector Host Distribution Febrile illness, rash, arthralgia Mosquito Primates, humans Africa, India, Southeast Asia O’nyongnyong (ONN) Febrile illness, rash, arthralgia Mosquito Primates Africa Sindbisc (SIN) Febrile illness, rash, arthralgia Mosquito Birds Nothern Europe, Africa, Asia, Australia Semliki Forest Febrile illness, rare encephalitis Mosquito Birds Africa FIGURE Alphavirus transmission. Virus abbreviations: Chik, chickungunya; RR, Ross River; May, Mayaro; ONN, O'nyong-nyong; SIN, Sindbis; EEE, eastern equine encephalitis; VEE, Venezuelan equine encephalitis. Pathogenesis of alphaviruses Symptoms : EEE Most people have no symptoms Central Nervous system symptoms develop 4-10 days after being bitten Sudden onset of fever, muscle aches, headache May progress to more severe symptoms such as seizure and coma (encephalitis) 30 to 50% of patients with encephalitis die of the disease Structure of Flaviviruses Principal medically important flaviviruses Virus Antigenic Clinical Syndrome Vector Host Dengue (DEN) Febrile illness, rash, hemorrhagic fever, shock syndrome Mosquito Humans Tropics, worldwide Yellow fever (YF) Hemorrhagic fever, hepatitis Mosquito Primates, humans Africa, South America St. Louis encephalitis (SLE) Encephalitis Mosquito Birds Distribution Americas Principal medically important flaviviruses Virus Antigenic Clinical Syndrome Vector Host Distribution Japanese encephalitis (JE) Encephalitis Mosquito Pigs, birds India, China, Japan, South-East Asia Febrile illness Mosquito Birds Africa, Middle East, Europe Encephalitis Tick Rodent West Nile Tick-borne encephalitis (TBE) Europa, Asia Principal medically important flaviviruses Virus Antigenic Clinical Syndrome Vector Host Distributio n Omsk hemorrhagic fever Hemorrhagic fever Tick Muskrats Siberia Kyasanur Forest disease (KFD) Hemorrhagic fever Tick Rodents India primates Tick-born encephalitis virus Human infection with both mosquito-borne and tick-borne flaviviruses is initiated by deposition of virus through the skin via the saliva of an infected arthropod (Fig). Figure. Pathogenesis of flaviviruses. Japanese Encephalitis First discovered and originally restricted to Japan. Now large scale epidemics occur in China, India and other parts of Asia. Most human infections are subclinical: the inapparent to clinical cases is 300:1 In clinical cases, a life-threatening encephalitis occurs. The disease is usually diagnosed by serology. No specific therapy is available. Since Culex has a flight range of 20 km, all local control measures will fail. An effective vaccine is available. Transmitting WNV infection Symptoms : West Nile virus Most people do not develop symptoms An estimated 20% become ill 3-15 days after being bitten Mild illness: fever, headache, body aches, and sometimes skin rash and swollen glands An estimated 1 in 150 persons infected develop a more severe form of the disease West Nile encephalitis: inflammation of the brain, high fever, stiff neck, stupor, disorientation, coma, tremors, convulsions, muscle weakness, and paralysis; few cases have been fatal Yellow fever Yellow Fever Flavivirus, mainly found in West Africa and S America Yellow fever occurs in 2 major forms: urban and jungle (sylvatic) yellow fever. Jungle YF is the natural reservoir of the disease in a cycle involving nonhuman primates and forest mosquitoes. Man may become incidentally infected on venturing into jungle areas. The urban form is transmitted between humans by the Aedes aegypti mosquito Classically Yellow Fever presents with chills, fever, and headache. Generalized myalgias and GI complaints (N+V). Some patients may experience an asymptomatic infection or a mild undifferentiated febrile illness. Yellow Fever After a period of 3 to 4 days, the more severely ill patients with a classical YF course will develop bradycardia (Faget's sign), jaundice, and haemorrhagic manifestations. 50% of patients with frank YF will develop fatal disease characterized by severe haemorrhagic manifestations, oliguria and hypotension. Diagnosis is usually made by serology There is no specific antiviral treatment An effective live attenuated vaccine is available against yellow fever and is used for persons living in or traveling to endemic areas. Dengue fever Dengue Dengue is the biggest arbovirus problem in the world today with over 2 million cases per year. Dengue is found in SE Asia, Africa and the Caribbean and S America. Flavivirus, 4 serotypes, transmitted by Aedes mosquitoes which reside in water-filled containers. Human infections arise from a human-mosquitoe-human cycle Classically, dengue presents with a high fever, lymphadenopathy, myalgia, bone and joint pains, headache, and a maculopapular rash. Severe cases may present with haemorrhagic fever and shock with a mortality of 5-10%. (Dengue haemorrhagic fever or Dengue shock syndrome.) Dengue Dengue haemorrhagic fever and shock syndrome appear most often in patients previously infected by a different serotype of dengue, thus suggesting an immunopathological mechanism. Diagnosis is made by serology. No specific antiviral therapy is available. Prevention of dengue in endemic areas depends on mosquito eradication. The population should remove all containers from their premises which may serve as vessels for egg deposition. A live attenuated vaccine is being tried in Thailand with encouraging results. Bunyaviridae is a family of arthropod-borne or rodent-borne, spherical, enveloped RNA viruses. Bunyaviruses are responsible for a number of febrile diseases in humans and other vertebrates. They have either a rodent host or an arthropod vector and a vertebrate host. Human diseases Caused by Viruses of the Family Bunyaviridae Genus and Group Virus Disease Vector Distributio n Fever Mosquito Africa Bunyavirus Bunyamwera Bunyamwera Bwamba Bwamba Fever , Rash Mosquito Africa California California encephalitis Enceph a-litis Mosquito North America Simbu Shuni Fever Mosquito Africa, Asia Human diseases Caused by Viruses of the Family Bunyaviridae Genus and Group Virus Disease Vector Distribution Phlebovirus Phlebovirus fever Alenquer Fever Unknown South America Naples Fever Sand fly Europe, Asia, Africa Rift Valley Fever Fever, encephalitis, hemorrhagic fever, blindness Mosquito Africa Sicilian Fever Sand fly Europe, Africa, Asia Human diseases Caused by Viruses of the Family Bunyaviridae Genus and Group Virus Disease Vector Distribution Nairovirus CrimeanCongo Nairobi sheep disease CrimeanCongo hemorrhagic fever Hemorrhagic fever Tick Nairobi sheep disease Fever Tick Africa, Asia Africa, Asia Human diseases Caused by Viruses of the Family Bunyaviridae Genus and Group Virus Disease Vector Distribution Hantavirus Hataan Hantaan HFPS (hantavirus pulmonary syndrom) Rodent Asia Puumala HFPS Rodent Asia Sequl HFPS Rodent Asia, Europe Human diseases Caused by Viruses of the Family Bunyaviridae Genus and Group Virus Disease Vector Distributi on Genus unassigned Bangui Fever, rash Unknown Africa Bhanja Fever, encephalitis Tick Africa, Europa, Asia Issk-kul Fever Tick Asia Kasokero Fever Unknown Africa Nyando Fever Mosquito Africa Tataguine Fever Mosquito Africa Wanowrie Fever, hemorrhage Tick Middle East, Asia FIGURE. Pathogenesis of bunyavirus infections. Humans are dead-end hosts of most bunyaviruses; however, the blood of Crimean-Congo hemorrhagic fever patients may be highly infectious. Signs of Crimean-Congo Hemorrhagic Fever Diagnosis Serology - usually used to make a diagnosis of arbovirus infections. Culture - a number of cell lines may be used, including mosquito cell lines. However, it is rarely carried out since many of the pathogens are group 3 or 4 pathogens. Direct detection tests - e.g detection of antigen and nucleic acids are available but again there are safety issues. Prevention Surveillance - of disease and vector populations Control of vector - pesticides, elimination of breeding grounds Personal protection - screening of houses, bed nets, insect repellants. When possible, wear protective clothing while outdoors. Vaccination - available for a number of arboviral infections e.g. Yellow fever, Japanese encephalitis, Russian tick-borne encephalitis Treatment No specific therapy Arboviral encephalitis treated by hospitalization, intravenous fluids, respiratory support, prevention of secondary infections, and good nursing care