Figure 1. Total number of reported cases of human
advertisement

AJ-3 FINAL REPORT
Mapping Especially Dangerous Pathogens in Azerbaijan
Jason K. Blackburn, Ian T. Kracalik, Lillian R. Morris, Allison Schlak
Spatial Epidemiology & Ecology Research Laboratory, Department of Geography, Emerging Pathogens
Institute, University of Florida, Gainesville, FL
Rakif Abdullayev, Ayden Talibzade, Rita Ismayilova, Narmin Ustun
Anti-Plague Station, Baku, Azerbaijan
Merhiban Baghirova, Mezahir, Climent Asadov
State Veterinary Service, Baku, Azerbaijan
Martin Adams, Edith Marshall, Sonya Narodny
Bechtel National, Inc
Contents
Project Summary........................................................................................................................................... 3
AJ-3 Project Background ............................................................................................................................... 8
Background/Justification .......................................................................................................................... 8
Chapter 1: Analyzing the spatial and temporal distribution of human anthrax in Azerbaijan during the
period 1983 to 2010.................................................................................................................................... 10
Introduction ............................................................................................................................................ 10
Materials and Methods........................................................................................................................... 10
Results ..................................................................................................................................................... 11
Discussion................................................................................................................................................ 15
References .............................................................................................................................................. 16
Chapter 2: Mapping hotspots of anthrax and genetic diversity of Bacillus anthracis in Azerbaijan .......... 17
Introduction ............................................................................................................................................ 17
Methods .................................................................................................................................................. 17
GIS data ............................................................................................................................................... 17
Anthrax hotspot mapping ................................................................................................................... 17
GARP ecological niche modeling: Presence-Only ............................................................................... 18
MLVA-25 Genotyping .......................................................................................................................... 19
Results ..................................................................................................................................................... 19
Discussion................................................................................................................................................ 23
Chapter 3: Identifying areas of plague habitat in Azerbaijan: Comparing ecological modeling techniques
to provide a better estimation of geographic suitability ............................................................................ 25
Introduction ............................................................................................................................................ 25
Methods .................................................................................................................................................. 25
GIS data ............................................................................................................................................... 25
Ecological modeling approaches: Presence/Absence ......................................................................... 26
Logistic Regression in R ....................................................................................................................... 26
Random Forest in R ............................................................................................................................. 26
GARP ecological niche modeling: Presence-Only ............................................................................... 26
Logit-Only Models ............................................................................................................................... 26
Superset .............................................................................................................................................. 26
Model accuracy assessment ............................................................................................................... 26
Model geographic comparisons.......................................................................................................... 26
Discussion................................................................................................................................................ 32
Chapter 4: Measuring inter-annual dynamics of Low-land Plague Focus in Azerbaijan using historical
maps and STAMP ........................................................................................................................................ 34
Introduction ............................................................................................................................................ 34
Materials and Methods........................................................................................................................... 34
Discussion................................................................................................................................................ 35
Chapter 5: Mapping human tularemia in Azerbaijan using historical data ................................................ 49
Chapter 6: Spatial patterns of livestock brucellosis in Azerbaijan 2002 to 2010........................................ 50
Introduction ............................................................................................................................................ 50
Materials and Methods........................................................................................................................... 50
Results ..................................................................................................................................................... 51
Discussion................................................................................................................................................ 51
References .............................................................................................................................................. 52
Chapter 7: The status of zoonoses in Azerbaijan during Soviet and post-Soviet governance: Analyzing
space-time patterns of human brucellosis and anthrax ............................................................................. 58
Introduction ............................................................................................................................................ 58
Materials and Methods........................................................................................................................... 58
Results ..................................................................................................................................................... 58
Discussion................................................................................................................................................ 59
References .............................................................................................................................................. 59
Chapter 8: Analyzing the spatial and temporal distribution of human brucellosis in Azerbaijan (1995 2009) using spatial and spatio-temporal statistics ..................................................................................... 66
Abstract ................................................................................................................................................... 66
Introduction ............................................................................................................................................ 66
Methods .................................................................................................................................................. 67
Results ..................................................................................................................................................... 70
Discussion................................................................................................................................................ 71
Limitations .............................................................................................................................................. 73
Conclusion ............................................................................................................................................... 74
References .............................................................................................................................................. 74
Project Summary
The AJ-3 project was a two-year GIS-centric CBR project focused on mapping and modeling the spatiotemporal and ecological patterns of four important bacterial zoonoses in Azerbaijan. The project was a
joint collaboration between the Republican Anti-Plague Station, Baku (APS), the State Veterinary
Service, Baku (SVS), and the Spatial Epidemiology & Ecology Research Laboratory, University of Florida
(SEER Lab). Working with historical data from each Azeri institute, this project developed spatiotemporal databases on anthrax, Bacillus anthracis, plague, Yersenia pestis, tularemia, Francisella
tularensis, and brucellosis, Brucella spp.. Over the course of an ~18 month project development grant
(PDG) period and a two-year project, geospatial data were developed and analyzed on each disease
system. At the same time, AJ-3 provided geospatial technology (GPS units) and GIS software (GIS
infrastructure and analytical tools) to each APS and SVS and extensive GIS training through a series of
engagements in Bishkek, Kyrgyzstan at the Kyrgyz Consortium for GIS Excellence (KCGE; Introductory GIS
for APS and SVS and Intermediate GIS for APS), the SEER Lab, and in-country site visits to each institute
by SEER Lab team members.
The goal of GIS training activities was to establish fundamental (sustainable) GIS skills for a core group of
researchers at APS and SVS and integrate real world application of GIS skills to institute duties using
important historical data on select agent diseases. GIS training was achieved during 7 in-country visits
between 2008 – 2012, 2 visits by APS (2008) and 1 visit by SVS to KCGE (2012), and 2 visits by APS
personnel to SEER Lab (2009, 2010).
Computers and software were provided to APS and SVS by the integrating contractors in 2008 (APS) and
2012 (SVS). High speed PCs were provided to each institute with ArcGIS (ESRI, Redlands, CA) as the
primary GIS software platform. All training sessions included work in the ArcGIS environment. In
addition, SEER Lab personnel provided training in a number of open source geospatial statistical
packages, including SaTScan (www.satscan.org), GeoDa (geodacenter.asu.edu) and DesktopGARP. SEER
Lab provided extensive training documentation on each statistical and GIS technique over the course of
training visits and email correspondence. In 2012, SEER Lab also began Skype-based meetings with SVS
to bridge the gap between collaborator visits to Baku.
At the same time, AJ-3 had a series of research objectives that can be summarized as establishing the
spatio-temporal baselines and associated ecology of the four diseases using GIS, spatial statistics, and
ecological modeling. Over the course of the project datasets for each disease were derived from
extensive efforts to catalog and digitize archival data at each institute. APS provided human-related data
on the occurrence of human anthrax and brucellosis for several decades and an extensive record of
pathogen isolation for B. anthracis, Y. pestis, and F. tularensis from pathogen passport records.
Brucellosis data were mapped from serological surveys conducted by each APS and SVS.
In addition to providing GIS training and research support, the project supported collaborator travel
costs to several conferences and publication costs for journal articles, allowing the project to showcase
research efforts in the international GIS and public health communities and receive peer-review
feedback and publication on project results.
In-country training sessions and research goals are summarized in Table 1.
Table 1. In-country site visits to APS and SVS by SEER Lab personnel.
AJ Site Visits
Objectives
30 June 2008 - 01 July 2008
First trip of PDG: GIS research and Data development
28 September 2009 - 2 October 2009
Conducted training GIS using LISA module and data
development of archival materials including: plague,
anthrax, and brucellosis
25 October 2010 - 29 October 2010
GIS training and development of human brucellosis
database. Field training with a focus on mapping plague
habitat in the Gobustan region
25 April 2011 - 29 April 2011
Compiling historical plague and tularemia isolate data
from archival records into a GIS. Field training with a
focus on mapping plague habitat across multiple
historical foci.
12 September 2011 - 16 September 2011
GIS training on space-time analytical techniques using
moving baselines with a focus on analyzing monthly
human brucellosis data.
27 April 2012 - 05 May 2012
Data development and GIS training of veterinary staff at
SVS. Analysis of human anthrax and brucellosis data at
APS. Field trip to Gobustan to map plague focus.
27 August 2012 - 31 August 2012
Advanced training using the STAMP analysis on plague
pathogen passport data. GIS training at SVS using
livestock brucellosis data on cattle and sheep.
Travel to conferences is summarized in Table 2 along with the work that was presented. In total, AJ-3
produced 10 conference papers co-authored by APS/SEER, APS/SVS/SEER, SVS/SEER and representing at
least some data on each of the four disease systems.
Table 2. Papers presented at international conferences by AJ-3 personnel.
Conferences
Abstract Title
American Association of Geographer 2010
Entomological Society of America 2010
(Co-presented as TADR Vector and AJ-3)
Brucellosis International Research Conference 2011
American Society of Tropical Medicine and Hygiene
2011
Spatio-temporal clustering of human anthrax outbreaks
in Azerbaijan, 1937-1998, using SaTScan
Preliminary Mapping of Distribution of Medically
Important Ticks in Azerbaijan in Support of the Defense
Threat Reduction Agency’s Biological Threat Reduction
Program
Analyzing the spatial and temporal distribution of
human brucellosis in Azerbaijan during the period 1983
to 2009: A comparison of gridded population data and
smoothing techniques
Spatio-Temporal Patterns of Emerging Human
Brucellosis Clusters in Azerbaijan During the Period
2000 to 2010 Using Varying Baseline Expectations of
Occurrence
General Meeting of the American Society for
Microbiology 2011
Analyzing the spatial and temporal distribution of
human anthrax cases in Azerbaijan 1983 to 2010
International Congress on Infectious Diseases 2012
The Status of Zoonoses in Azerbaijan during Soviet and
Post-Soviet Governance: Analyzing Space-Time patterns
of Human Brucellosis and Anthrax
DTRA Science Review 2012
Identifying areas of plague habitat in Azerbaijan:
Comparing ecological modeling techniques to provide a
better estimation of geographic suitability
DTRA Science Review 2012
Mapping hotspots of anthrax and genetic diversity of
Bacillus anthracis in Azerbaijan
American Society of Tropical Medicine and Hygiene
2012
American Society of Tropical Medicine and Hygiene
2012
Brucellosis Research Conference 2012
Mapping hotspots of anthrax and genetic diversity of
Bacillus anthracis in Azerbaijan
Measuring inter-annual dynamics of the TransCaucasian Low-land Plague Focus in Azerbaijan using
historical maps and Spatial-Temporal Analysis of
Moving Polygons (STAMP)
Spatial patterns of livestock brucellosis in Azerbaijan
2002 to 2010
To date, AJ-3 has published a peer-reviewed paper on the spatio-temporal patterns and baseline of
human brucellosis at the rayon level in the international journal, BMC Infectious Diseases (Abdullayev et
al. 2012). In addition to this first paper, all chapters below (minus chapter 5 – due to limitation of
available data) are in the process of being expanded and drafted into manuscripts with efforts from each
institute. These papers will be submitted for publication by mid to late 2013.
This report provides an overview of the research completed on the AJ-3 project in a series of chapters.
To place these efforts in the context of the AJ-3 project goals, milestones from the project work plan are
presented below with indications of the chapters in this report that reflect those analyses or research
efforts. These milestones are presented in the order they appear in the AJ-3 Form A.
Historical veterinary data from Anthrax – vaccination records held by APS and
livestock incidence data held by RVL will be input into GIS data tables. At least
ten years of data (from 1975-2009) in each category will be input.
Historical veterinary data were provided by SVS for several decades. Detailed vaccination data records
were not available due to the restructuring of the SVS in recent years. However, outbreak data were
available and mapped during the course of this project. SEE CHAPTER 2.
Enter passport data from current museum strains (at least 50) into PACS and
extract needed GIS information to incorporate these into the overall historical
analysis.
PACS was not used during AJ-3. However, nearly 1000 pathogen passports were identified from the APS
museum/library, scanned, translated, and geolocated to map pathogen distributions. Chapter 3 provides
a detailed analysis of plague passports. Chapter 5 provides a review of the relatively few tularemia
passports, and Chapter 2 provides an analysis of anthrax-related passports, specifically those with
associated strains that were genotyped by WRAIR as part of the DTRA ChemBio funded Strain
Characterization project and provided the AJ-3 for modeling.
Combined analysis of Anthrax human outbreak data prepared during AJ -3 with
veterinary data.
During the 2012 working year, data from APS and SVS were combined in a single analysis of human an
animal livestock using a hotspot analysis (Chapter 2). Data from APS and SVS on anthrax reports were
mapped by decade and illustrated side-by-side to evaluate the overlap between hotspots in each
reporting system (human or animal). This work was combined with an ecological niche model of Bacillus
anthracis to compare the habitats that might support spore survival and compare to ecological hotspots.
Ecological niche models were paired with MLVA-25-based genotype results from an effort lead by M.
Nikolich and a ChemBio project. Anthrax hotspots were presented along with the phylogeny of B.
anthracis compared to the global diversity to place AJ into the regional context of strain diversity and
match that to outbreak histories. We provide a spatio-temporal history of anthrax and the ecological
niche-based maps to define areas of priority for surveillance.
Input at least 10 years of APS human Brucellosis data (selected from 1982 -2009)
into GIS data tables
Human brucellosis data are presented in chapters 7 and 8. Data were provided at the rayon data for the
period 1983-2009, giving us an opportunity to evaluate pre-/post- independence incidence rates and
spatio-temporal patterns. We provide a baseline of human brucellosis in Chapter 8 that can function to
inform surveillance efforts.
Geospatial and temporal analysis of human Brucellosis data set
See Chapter 7 and Chapter 8 and milestone above.
Construct a Geodatabase from maps digitized during the AJ -3 PDG
During the AJ-3 PDG period, we identified a series of plague yearbooks from the Soviet period that
provided annual distributions of plague vectors and hosts. We developed an extensive GIS of these data
and provide a thorough analysis of space-time changes in Chapter 5. We used the STAMP GIS toolbox to
analyze areas of high mammal abundance historically that may identify important areas in Azerbaijan for
plague surveillance today.
Data review with collaborators to select time periods to cover with Brucella,
Tularemia, and Plague GIS data entry and analysis
In this report we provide data on each disease in the Form A.
Input selected 10 years of RVL livestock Brucellosis data (should have maximum
overlap with human data timeframe) into GIS data tables
Working with SVS, we provided a 10-year database of livestock brucellosis from 2002-2012. See Chapter
6 of this report. We provide a spatio-temporal analysis of the disease over 3 consecutive 3-year periods.
We also describe the current livestock surveillance system.
Combined geospatial and temporal analysis of Brucellosis in Azerbaijan
A large portion of this report is dedicated to this milestone and see above.
Input selected 10 years of APS human Plague and Tularemia data into GIS data
tables
Plague data from pathogen passports are summarized and spatially modeled in Chapter 3 of this report.
Tularemia data are provided in Chapter 5.
Geospatial and temporal analysis of human Plague and Tularemia data set
Chapters 3 and 4 provide extensive modeling of plague data. Chapter 5 provides the limited tularemia
data available. We map tularemia data, but we did not analyze it for patterns due to sample size
limitations.
Input corresponding 10 years of APS reservoir and vector surveillance data into
GIS data tables
During this project, we mapped 13 years of data from historical records on plague. See Chapter 4 of this
report.
Take data reported by the TADR network through EIDSS and build contemporary
GIS data sets on Plague, Tularemia, Brucellosis, and Anthrax
We were unable to link TADR/EIDSS and AJ-3 data in this project. However, anthrax and brucellosis data
are contemporary through 2011.
Take data generated in AJ-3.2 and build contemporary GIS data sets on Plague
and Tularemia
We were limited to historical data on plague and tularemia in this project. However, we did conduct GIS
field mapping exercises that confirmed that historical plague foci are still active for primary host
mammals (e.g. Meriones libycus), though we have no associated pathogen data, as it was beyond the
scope of the AJ-3 project.
Update analysis of Anthrax to compare historical and contemporary data
Chapter 2 provides an up-to-date analysis of anthrax in the country for humans and livestock.
Update analysis of Brucella to compare historical and contemporary da ta
Chapter 8 provides an up-to-date analysis of human brucellosis and Chapter 6 provides an update on
livestock brucellosis over the past decade.
AJ-3 Project Background
Background/Justification
Understanding the spatial patterns of infectious diseases can provide insight into
ecological/environmental, socio-economic, and other risk factors that promote outbreaks or disease
persistence and potential improvements for control or eradication strategies. Geographic information
systems (GIS) are increasingly being used to analyze geographical distribution of diseases as well as
relationships between pathogenic factors (causative agents, patients, vectors and hosts) and their
geophysical environments. A GIS can be simply defined as the personnel, computer hardware and
software, database technologies, and spatial analyses that can be integrated to evaluate spatial
relationships between disparate (and often times idiosyncratic) data sets to better understand the
geographic structure of biological or geophysical phenomena. Basic and analytical applications of GIS
in epidemiology can help in visualizing (mapping) and analyzing geographic distributions of diseases
through time, thus revealing geographic patterns, spatio-temporal trends, and relationships that
would be more difficult or obscure to discover in tabular or other formats.
These analyses can generate disease baselines (quantitative expectations of disease events across
discrete geographic areas and time periods) against which monitoring systems can calibrate and
better prepare for prevention and response operations. Understanding the spatial spread and
temporal dynamics of an outbreak is central to the design of surveillance, prevention, control, and
response strategies. Well-trained personnel equipped with GIS technologies and analyses provide a
means to meet these needs and can be centrally placed as a powerful decision support team/tool box
within epidemiology and emergency preparedness.
An expansive archive of historical records remains relatively intact at the Republican Anti-Plague
Station (APS) in Baku, Azerbaijan. Examination of these records through the AJ-3 Project Develop
Grant (PDG) period has revealed potentially valuable historical disease surveillance data for several
especially dangerous pathogens ranging from approximately 1930 until the late 80s. Data since the
collapse of the Soviet Union is also maintained in this archive, though the data points collected during
that time are sparse due to the resource vacuum for APS in the post Soviet rebuilding period. The
availability of this archive presents a unique opportunity to map historical especially dangerous
pathogen prevalence within the country of Azerbaijan as well as leverage the core of epidemiologists
who are equipped, trained and experienced in the utilization of GIS for disease surveillance and
Geographic Information Science (GISc) methodologies for spatial analysis and statistics for disease
ecology. The records are expected to provide information on Y. pestis, F. tularensis, Brucella species,
and B. anthracis. It is less likely but nevertheless possible that some records will report smallpox
cases, Crimean Congo hemorrhagic fever (CCHF), tick borne encephalitis (TBE), or fevers of unknown
origin. If other records of interest to BTRP are identified during the course of the project DTRA and
the US collaborators will be made aware of their existence and the data will be made available. A
unique opportunity exists to exploit this data archive, in that one of the project participants, Dr.
Ayden Talibzade has intimate knowledge of the archive contents, having worked with the records
since 1961.
Chapter 1: Analyzing the spatial and temporal distribution of human
anthrax in Azerbaijan during the period 1983 to 2010
Introduction
Recently it has been suggested that the impact and burden of anthrax has not been fully realized
(Fasanella et al. 2010). Anthrax is a soil-borne zoonosis caused by the Gram-positive, spore forming
bacterium Bacillus anthracis, which primarily infects livestock and wildlife and secondarily afflicts
humans (Hugh-Jones and Blackburn 2009). Despite the existence of an efficacious livestock vaccine,
anthrax remains a problem in many developing countries including former states of the Soviet Union,
which have undergone dramatic changes in their public and veterinary health infrastructure (Hugh-Jones
1999).
Economic and political changes in Azerbaijan brought on by the collapse of the Soviet Union have
resulted in decreased funding for health surveillance (Saleem et al. 2010). In order to better understand
the spatial and temporal distribution of human anthrax in Azerbaijan geospatial analytical techniques
and geographic information systems (GIS) mapping were employed. There were three primary
objectives of this study: 1) to describe the spatial and temporal distribution of human anthrax in
Azerbaijan 2) to identify the potential presence of spatial clusters of the disease and 3) to analyze the
efficacy of using alternative population datasets to generate risk estimates.
Materials and Methods
Anthrax is a reportable infectious disease in Azerbaijan. Surveillance and documentation of health
events with in the country are undertaken by their surveillance and diagnostic laboratory known as the
Anti-Plague Station (APS), which is divided into five reporting zones. Each of the five reporting zones has
a Regional APS (RAPS) office that responds to health inquiries in order to obtain laboratory samples and
verify any diagnosis. The total number of new cases per year were aggregated to the district level and
grouped into nine equal three year periods with the exception of period 9 which contained four years of
data. Average incidence was calculated per district for each of the nine periods.
Average Incidence per 100,000 individuals was mapped at the district level to highlight any spatial
changes in risk over time. Smoothed risk estimates were also calculated using the Spatial Empirical
Bayesian smoother (SEBS) in the GeoDa software package (Anselin et al. 2006). Cluster analysis was
performed using the Local Moran’s I statistic a local indicator of spatial autocorrelation (LISA) in the
GeoDa software package using smoothed rates. The statistic can identify hotspots as well as spatial
outliers, or in this case individual districts, that vary disproportionately from the global mean. Rayons
are deemed to be not significant or a cluster of either High-High, Low-Low, High-Low, or Low-High values
relative to neighboring rayons. The null hypothesis states that there is no spatial autocorrelation or
association of anthrax outbreaks between rayons. The local Moran’s I statistic is written as (following
Anselin, 1995):
𝐼𝑖 = 𝑍𝑖 ∑ 𝑤𝑖𝑗 𝑍𝑗
𝑗
where Ii is the statistic for district i, Zi is the difference between the smoothed incidence at i and the
mean anthrax outbreaks for Azerbaijan, Zj is the difference between anthrax outbreaks at j and the
mean for Azerbaijan. Wij is the weights matrix that only considers neighbors that share a common
border or vertices (in the Rook contiguity case Wij is 1/n if a rayon shares a border or a vertex and zero
otherwise). The statistic was implemented in GeoDa 0.9.5-i (Anselin et al., 2006) using a Queen
contiguity matrix, and 999 permutations at an α < 0.05. Due to the discontinuity between rayons in
Azerbaijan Nakchivan was excluded from the Spatial Empirical Bayes analysis and the Local Moran’s I
analysis. Furthermore, in order to evaluate the efficacy of using alternative population estimates to
calculate risk at the district level, gridded population datasets for the year 2006 were obtained from
GPW3 and Hyde. Incidence rates were calculated using gridded population for the year 2006
aggregated to the district level using the zonal statistics routine in ArcGIS v9.3. The percent difference
between risk estimates generated by the APS in Baku was compared to GRUMP and HYDE estimates.
Results
Reported cases of human anthrax show inter annual variability with a maximum number of cases of 76
in 1996 and no cases reported in 1983, 2002, 2005, and 2010 (Figure 1). During the period 1983 to 2010
approximately 490 human anthrax cases were recorded across Azerbaijan (Figure 1). Age of cases
illustrates a disproportionate burden of the disease with individuals age 30 to 59 more heavily afflicted.
Spatial patterns of average incidence estimates portray spatial variation in risk among districts (Figure
3). Smoothed spatial estimates depict spatial patterns of varying risk throughout the country compared
with the crude estimates. Application of the SEBS illustrates the affects the algorithm had on adjusting
the risk estimates for each time period (Figure 4). In general, the smoothing algorithm was successful in
shrinking the risk estimates for each of the nine equal, three year time periods, thereby limiting the
effects of outliers. Furthermore, approximately one third of the districts in Azerbaijan contained 80% of
all the reported cases human anthrax during the study period. Figure 5 depicts the spatial cluster
analysis and four different types of spatial autocorrelation (High–High), (Low-Low), (Low-High), or (HighLow) detected during the study period. High-High clusters were primarily concentrated in the west in
periods 1-5, and in the east during periods 6-9.
Results from the gridded population incidence rate estimates shows a significant amount of variation in
risk when compared to the Azeri estimates (Figure 6). The 2006 HYDE and 2006 GPW3 risk estimates
both illustrate an underestimation of risk in the west and an overestimation of risk in the east when
compared to the 2006 Azeri risk estimates. The difference in risk for HYDE compared to the Azeri’s
estimate ranged from 0 to -5 cases per 100,000 while GPW3 compared to the Azeri’s estimate ranged
from +2.6 to -4.97 cases per 100,000. Comparison of the estimates for HYDE and GPW3show spatial
differences between the two estimates in the west and east.
80
14
70
Cases
12
60
CASES
8
40
6
30
4
10
2
0
0
1983
1984
1985
1986
1987
1988
1989
1990
1991
1992
1993
1994
1995
1996
1997
1998
1999
2000
2001
2002
2003
2004
2005
2006
2007
2008
2009
2010
20
Incidence
10
50
YEAR
Figure 1. Total number of reported cases of human anthrax by year shown by the black bars and the
incidence rate per 1,000,000 population indicated by the red line.
300
250
Cases
200
150
100
50
0
0-14
15-19
20-29
Age Group
Figure 2. Total number of human anthrax cases by age group.
30-59
> 60
Figure 3. Average incidence of human anthrax per 100,000 persons from 1983 to 2009 broken into 9,
three year periods. Inset A displays the crude cumulative incidence risk by districts across Azerbaijan
while inset B displays Spatial Empirical Bayes smoothed estimates. Darker colors represent higher risk
estimates while lighter colors represent lower risk estimates. Numbers indicate the corresponding three
year time period, with group 1 representing the period 1983 to 1985 and so on.
Figure 4. Boxplot comparing crude and Empirical Bayes smoothed risk estimates for each of the 9, three
year periods. Numbers indicate the corresponding three year time period from Figure 3 with group 1
representing the period 1983 to 1985 and so on. The boxplot displays the values of the 25th, 50th and
75th percentiles. The whiskers extend to the most extreme data point.
Figure 5. Local Moran’s I clusters across Azerbaijan for each time period are shown in with dark red
portraying High-High areas of anthrax incidence, and pink High-Low areas.
Figure 6. Map displaying the difference in risk estimates for the year 2006 comparing HYDE to the Azeri
estimates, GPW3 to the Azeri estimates and HYPDE to GPW3. Districts showing a high percentage of
difference between risk estimates are shown in dark red while lower percent differences are shown in
dark purple.
Discussion
Azerbaijan has undergone significant political and economic changes in the last twenty years. Changes
in the funding and organization of public health management and surveillance have most likely
contributed to problems with surveillance and reporting of the disease in Azerbaijan (Clark) et al. 2011).
Analyzing the spatial and temporal distribution of diseases may aid in identifying changes in the
epidemiology or status of a disease. The results from this analysis depict a varying spatial and temporal
distribution of risk among districts.
Since anthrax is not a contagious disease cluster of human cases are most likely a result of shared food
sources or product of similar socio-cultural practices that include the butchering or rendering of animals.
Clusters identified by the LISA analysis in the eastern part of the country could potentially represent an
area that requires additional surveillance. Furthermore, the use of gridded population data to map
populations at risk estimates may be useful for situations when no clear estimates of population can be
derived, but results from this analysis show a high level of variation in risk.
The analyses presented in this study represent the first attempts to use GIS and spatial analysis to
describe patterns of human anthrax in Azerbaijan. Incorporating new techniques in order to better
understand the epidemiology of the disease mark a new phase in the public health infrastructure of the
country. Future efforts of public health management and researchers may now try to incorporate data
on livestock in conjunction with human data in order to better understand the transmission dynamics of
anthrax in Azerbaijan.
References
Anselin, Luc, I. Syabri, and Y. Kho. 2006. GeoDa: An introduction to spatial data analysis. Geographical
Analysis. 38:5–22.
Beyer, H. L. 2004. Hawth's Analysis Tools for ArcGIS. Available at http://www.spatialecology.com/htools.
Clark, D. et al. 2011. Under-utilization of health care services for infectious diseases syndromes in rural
Azerbaijan: A cross-sectional study. BMC Health Services research.
Hugh-Jones, M., 1999: 97 global anthrax report. J Appl Microbiol, 87, 189-191.
Hugh-Jones, M. and J. Blackburn, 2009: The ecology of Bacillus anthracis. Molecular Aspects of Medicine,
30, 356-367.
Jacquez, Geoffrey M. 1996. A k nearest neighbor test for space-time interaction. Statistics in Medicine.
15:1935-1949.
Kulldorff, Martin. 1997. A spatial scan statistic. Communications in statistics. 26(6):1481-1496.
Saleem et al. 2010 anthrax: a remerging zoonosis. Veterinary Microbiology. 3-4(140) : 392-398.
Chapter 2: Mapping hotspots of anthrax and genetic diversity of Bacillus
anthracis in Azerbaijan
Introduction
Anthrax is an acute zoonotic disease of domestic and wild herbivores, with secondary cases in humans,
usually related to spillover dynamics associated with handling animal carcasses. Anthrax has a historical
existence in Azerbaijan and remains a concern today due to inadequacies in public health and veterinary
surveillance (Hugh-Jones, 1999). Though Azerbaijan has a long historical record of human and animal
outbreaks, limited information is available on the geography or local ecology of the disease or genetic
relationships between local strains and global diversity. The disease is caused by the bacterium Bacillus
anthracis, a spore-forming organism with potential for persistence and reoccurrence under specific soil
conditions and long-distance transmission events. Spatial techniques can be used to model such
ecological conditions.
This study combines spatial hotspot mapping of historical outbreaks with ecological niche models of the
pathogen to better understand historical distribution of disease and associated changes through time
(hotspot mapping) and the potential geographic distribution of environments that likely promote spore
survival (niche modeling). Together these analyses provide prospective on where the disease has
persisted across much of the last century, as well as those locations on the landscape where persistence
is linked to ecological conditions.
To further our understanding of the genetics of Bacillus anthracis, we provide the first genotyping effort
for strains from Azerbaijan using the multi-locus variable number tandem repeat analysis on strains
from the repository at the Azerbaijani Republican Anti-plague station shared with the Walter Reid Army
Institute of Research. Together these analysis provide an in-depth look at the spatio-temporal patterns
of anthrax reporting in both human and animal populations for Azerbaijan, as well as the environmental
characteristics associated with the genetic lineage we identified using MLVA.
Methods
GIS data
A GIS was constructed from historical records of known villages reporting human anthrax between the
years 1937 and 1998 and livestock anthrax between 1940 and 1999. Village locations were geocoded
using the Geonames database. The total number of years any village reported was generated by
summating all years for each location (Figure 1).
Anthrax hotspot mapping
Kernel density estimation (KDE) was performed in ArcGIS 10 to map the spatial distribution of anthrax
reports for each decade for each group. KDE is an interpolation technique for calculating weighted
densities of events over a gridded surface within a kernel, or spatial bin (Fotheringham et al. 2000).
Kernel density analysis was performed with the Spatial Analyst Extension for ArcGIS 10. ArcGIS employs
the quadratic kernel function described in Silverman (1986, p. 76, equation 4.5) and presented in
equation 1. Where h is the bandwidth, x-Xi is the distance to each village i in a given decade . K is the
quadratic kernel function, which is defined in equation 2. KDE is dependent on bandwidth, calculated
here as hopt (equation 3). Hotspots were defined following Nelson and Boots (2008) as the highest 25%,
10%, and 5% of density values.
𝒏
𝟏
𝒙 − 𝑿𝒊
𝒇(𝒙) =
∑𝑲(
)
𝒏𝒉
𝒉
𝒊=𝟏
𝑲(𝒙) =
𝟑
(𝟏 − 𝒙𝟐 ), |𝒙| ≤ 𝟏
𝟒
𝑲(𝒙) = 𝟎, 𝒙 > 𝟏
𝟏
𝒉𝒐𝒑𝒕
𝟐 (𝟒)
=[ ] 𝝈
𝟑𝒏
GARP ecological niche modeling: Presence-Only
We built GARP models using spatially unique locations of B. anthracis isolates. Since data were limited to
8 locations, we used all locations and relied on intrinsic model accuracy. Two sets of environmental
coverages were used (Table 1). Total commission of each experiment was reported.To evaluate the
spatial predictions of both model sets, we reclassified final model outputs based on 6 or better best
subset models. Final output models were summated and color coded.
Table 1. Environmental coverages used in GARP ecological niche modeling experiments.
Bioclimatic
Model
Soils
Model
Data source
Annual
mean
Bio 1
temperature(˚C)
X
X
www.WORLDCLIM.org
Annual temperature
Bio 7
range (˚C)
X
Annual
(mm)
Bio 12
X
Precipitation of the
Bio 13
wettest month (mm)
X
Variables
Name
precipitation
www.WORLDCLIM.org
X
www.WORLDCLIM.org
www.WORLDCLIM.org
www.WORLDCLIM.org
Precipitation of the
Bio 14
driest month (mm)
X
Elevation (m)
Alt
X
X
www.WORLDCLIM.org
Mean NDVI (no units)
wd0114a0
X
X
TALA (Hay et al. 2006)
NDVI
Amplitude
( no units)
Wd0114a1 X
Mean
soil
(no units)
Annual
pH
avg_pH
TALA (Hay et al. 2006)
X
FAO/IIASA/ISRIC/ISSCAS/JRC
Mean topsoil CaCO3
avg_caco3
(% weight)
X
FAO/IIASA/ISRIC/ISSCAS/JRC
Mean organic content
avg_OC
(% weight)
X
FAO/IIASA/ISRIC/ISSCAS/JRC
MLVA-25 Genotyping
Genetic diversity of B. anthracis was evaluated using the multi-locus variable number tandem repeat
analysis (MLVA) using a 25 primer set described by Lista et al. (2006). For this study, MLVA-25 results
from 3 isolates were combined with published values from Lista et al. (2006). Data analysis was done
using GeneMapper software (Applied Biosystems). Bins were assigned for each allele. Alleles falling
outside the bin was checked manually to determine if the peak was scored correctly. If an allele
consistently missed the bin it was scored against a reference sample containing the same allele. Once
the alleles were scored, we examined genetic relationships among the global strains using Unweighted
Pair Group Method with Arithmetic Averages (UPGMA) clustering analysis of the complete VNTR data
set. Distance matrices were generated in PAUP 4.0 and exported into the MEGA 5 software package for
dendrogram construction.
Results
Kernel density analyses are presented in Figure 2. Anthrax reporting varied across decades for both
groups, with greatest agreement between SVS-defined anthrax risk zones and KDE hotspots in the
1950s, 60s, 90s for human reports and 1960s, 70s, and 80s for veterinary reports. GARP models of B.
anthracis predicted areas in the north and south of the country, including many of the areas identified
as decadal hotspots, including areas outside of the 5-rayon risk zone (Figure 3). While purely exploratory
(due to limited isolate sample size), GARP models predicted a large proportion of the SVS risk zones,
with the exception of a rayon in the south of the country. MLVA-25 results suggest at least two
genotypes for 3 isolates collected from human patients between 1940 and 1967. All three isolates
belong to a single lineage following Lista et al. and were most closely related to isolates from
neighboring Iran. To date, partial data (17 of 25 markers) on an additional 14 isolates (all collected from
8 locations) suggests that all of the isolates are likely in this same lineage.
Figure 1. Village-level reporting of anthrax outbreaks by decade in the human (A) and livestock (B)
populations. Dot size represents number of years within the decade with reports, with increasing size
presenting a greater number of years reported.
Figure 2. Kernel density estimation-based hotspots of human (A) and livestock (B) anthrax by decade
from village-level reporting. Red areas reflect highest 25%, 10%, and 5% of KDE values. Black rayons
identify SVS-defined Zones of Anthrax Risk.
Figure 3. GARP best subset for the soils model (A), the bioclimatic model (B) and the agreement
between the two experiments based on a 6 model or better cutoff (C). Green dots are unique locations
of B. anthracis isolates used to build niche models. Yellow rayons are those defined as Anthrax Risk
Zones by AJ SVS.
Figure 4. UPGMA dendogram of MLVA-25 data illustrating clustering of the two Azerbaijani genotypes
(blue text) with the 67 genotypes reported by Lista et al. (2006)(black text). Bold black text identifies the
nearest genetic neighbors.
Discussion
This study provides the first effort to map anthrax persistence at the village level for Azerbaijan. Hotspot
analyses illustrate high levels of agreement with risk zones defined by SVS from a historical perspective,
but do not necessarily agree with the most recent decades reported. This may be a result of changes in
the surveillance post-Azerbaijani independence. This may also reflect changes in the endemicity of
anthrax as control and vaccination were established during this study period. The ecological niche
models provide a first effort to predict areas on the landscape where B. anthracis might persist and, as
suggested in other studies, can inform passive surveillance. For example, areas predicted with high
model agreement should have regional diagnostic capacity for B. anthracis even if the disease is rarely
seen. The hotspot analyses may assist in defining targeted control and surveillance priorities for
livestock. In the latter case, hotspots reflect known areas of repeat outbreaks. This study also provides
the first phylogenetic analysis of B. anthracis for the country. MLVA-25 results identified genotypes most
closely related to published Iranian strains, a southern neighbor with well documented anthrax, and a
wholly different lineage from work published out of Georgia, the northern neighbor. More work is
needed to evaluate these genetic and geographic relationships. Ultimately, linking these GIS-based
models with high resolution genotyping should provide the Azerbaijani public health system with the
ability to traceback outbreaks and better define zones of risk.
Chapter 3: Identifying areas of plague habitat in Azerbaijan: Comparing
ecological modeling techniques to provide a better estimation of
geographic suitability
Introduction
Plague is an acute flea-borne zoonotic disease caused by the bacterium Yersinia pestis. Although
reported human cases of the disease are rare in Azerbaijan several areas within the country have
persisted as foci for enzootic transmission, particularly in the central region of the country where the
Libyan gird, Meriones libycus, colonies may be abundant. However, decreased funding for surveillance
and eradication have made establishing current limits of plague foci expansion and contraction difficult;
therefore there is a need to identify areas that may support the pathogen to better provide public
health officials with modern estimates of potential geographic range.
Ecological modeling approaches, such as presence-absence techniques and presence-only ecological
niche modeling, are regularly employed to address such needs and produce maps of vector, host, or
disease distributions. Such approaches relate environmental data (such as remotely sensed climate
data) and species’ occurrence data (such as latitude/longitude coordinate pairs of where hosts or
vectors have been found) through the application of a fitted function, such as a logistic regression or an
iterative algorithm that fits logic strings. In particular, such approaches can provide estimates of under
sampled, or non-sampled, areas on the landscape by searching out areas on the landscape where nonrandom relationships between climate and occurrence points can occur. In other words, these
techniques fit these functions or relationships to the broader landscape to identify all possible areas
within the study boundary where conditions might support the pathogen or disease transmission.
There are a number of modeling tecniques available to predict the potential habitat of a pathogen,
vector or host species. While the ultimate goal of these techniques is similar, the methodologies and
associated predictions might vary greatly. In this paper, we aim to explore this issue and provide an
assessment of the differences in modeling techniques to understanding the potential distribution of
plague in Azerbaijan.
Methods
GIS data
Plague models were derived from historical pathogen pasmodsports located within the APS archives
that contained the location of plague isolates. This database was sub-divided by host species. M. libycus
models were also developed, as this was the most numerous host species represented. Geographic
locations of confirmed isolates were mapped to the nearest village location and assigned latitude and
longitude in ArcGIS v 9.3.1. In total, 130 unique plague isolates were geo-located across Azerbaijan.
Environmental variables were clipped to the geographic boundary of Azerbaijan and resampled to 0.1
degrees (~1 km2). Variables were tested for colinearity before model building. Variables are listed in
Table 1.
Ecological modeling approaches: Presence/Absence
We employed two presence/absence approaches. Pseudo absence data were generated using a 1km x
1km background grid cell for logistic and random forest models. Approximately 400 pseudo absence
points were randomly selected.
Logistic Regression in R
A step-wise logistic regression model was constructed in R. AIC and Homer & Lemeshow were used to
evaluate model fit.
Random Forest in R
Random forest models were built in R for each occurrence data set and models using the same training
data and variable set.
GARP ecological niche modeling: Presence-Only
Logit-Only Models
We built a modified GARP enm using only the logit rule type. To compare to P-A models, Logit-only
models used the same variable set and presence data set as the logistic and random forest models in R.
Because all GARP experiments are random walks, we used the best subset routine to reduce 100 models
down to 10 in each experiment.
Superset
We built traditional GARP models using all four rule types for each data set. Since supersets include
range rules, we replaced the categorical altitude variable with continuous altitude. We built equivalent
logit-only models to compare to the superset.
Model accuracy assessment
Occurrence data were split into 75% training/ 25% testing datasets in order to validate the model.
Model validation and accuracy were obtained through an area under the curve (AUC) score.
Model geographic comparisons
To evaluate the spatial predictions, we reclassified final model outputs based on 0.5 thresholds for P-A
models and 6 or better best subset models for GARP runs. Final output models were summated and
color coded.
Table 1. Variable list and inclusion for each of the models developed in this study. X indicates that the
variable was used in the development of that particular model.
Final Models
Variables
Name
Logistic/
Logit
Random
Forest
GARP
Superset
Superset/Logit
Annual
Mean
Temperature
Bio 1
X
X
X
X
Annual
Temperature
Range
Bio 7
Annual
Precipitation
Bio 12
Precipitation
of Wettest
Month
Bio 13
www.worldclim.org
Precipitation
of Driest
Month
Bio 14
www.worldclim.org
Elevation
Alt
Categorical
Elevation
Alt Cat
Source
www.worldclim.org
www.worldclim.org
X
X
X
X
X
X
X
X
Interaction
Interaction
Bio12 x Alt
X
X
X
X
Mean NDVI wd0114a0
X
X
X
X
NDVI Annual
Wd0114a1
Amplitude
www.worldclim.org
www.worldclim.org
www.worldclim.org
TALA (Hay et al.
2006)
TALA (Hay et al.
2006)
Figure 1. Geographic potential for Plague based on logistic regression (A), random forest (B), and GARP
logit-only (C) models using comparable variable data sets. Green and yellow dots reflect presence
training and testing points. Squares represent the absence data used.
Table 2. Sample sizes and accuracy metrics for each modeling approach. GARP Logit model metrics are
for models using the variable set used in P-A models. GARP superset models were not directly
comparable to other model approaches and should not be evaluated directly against other approaches.
Metric
Random GARP
Logistic
Random Forest LogitLogistic M.
Forest M.
Only
Plague libycus
Plague libycus Rules
only
only
Plague
GARP
GARP
LogitSuperset
Only M.
Plague
libycus
GARP
Superset
M.
libycus
N to build models
425
318
(105)a (62)a
425
(105)a
318
(62)a
105
62
105
62
N to test models
107
(27)b
107
(27)b
80
(16)b
27
16
27
16
Total Omission
55.60% 62.50% 11.10% 6.30%
33.30% 31.30% 11.10% 12.50%
Total Commission
9.50% 6.50% 6.10%
3.78%
9.96%
11.98% 18.76% 15.56%
AUC
0.83
0.91
0.73
0.73
a. Number of presence points used to build the
model
b. Number of presence points used to test the model
80
(16)b
0.84
0.93
0.73
0.77
Figure 2. Model agreement between the Logitistic from R, Random Forest, and GARP Logit-Only models.
Cutoffs: Logistic from R and Random Forest = 0.5, GARP Logit-Only 6 or more models from the best
subset.
Table 3. Parameter estimates from the logistic regression model for Plague developed using R.
Parameter Estimates
Variables
Coefficient
SE
Intercept
3.25
2.06
0.11
Bio 12
-9.05E -03
2.39E-03
<0.001
Bio 1
2.65E-02
7.70E-03
<0.001
Alt Cat
-6.28
1.41
<0.001
Wd0114a0
-3.32E-03
1.36E-03
<0.001
p-value
Interaction
1.30E-02
1.36E-03
<0.001
A
B
Figure 3. GARP Superset prediction of Plague data set using continuous Altitude variable (A).
Comparison of GARP Superset model and GARP Logit-Only model each using continuous Altitude (B).
Figure 4. Variable rankings from the Random Forest models for the M. libycus (A) and Plague (B). Note
the difference in variable contribution between each of the models. This may indicate a difference in
ecological conditions associated with the rodent host species versus all species that contribute to the
Plague model.
Discussion
Plague models differed among all four model routines, with similarities in western and eastern central
Azerbaijan across the three models using the same variable set (Figure 1). GARP Logit-only and Random
Forest both predicted disjunct areas in the south. The GARP superset predicted the broadest
geography, which is not unexpected from other studies comparing modeling approaches. GARP Logitonly models performed well in comparison to presence-absence models, despite the constraint that
absence data have may provide in P-A models. ALL GARP models had comparable AUC values, but broad
ranges of omission and commission. This difference in these metrics partially explains these similar AUC
scores (as AUC is ultimately described from these), illustrating why it is a difficult metric to evaluate
models. GARP Supersets cannot be directly compared to other models in Figure 1, but it did overpredict
compared all other modeling approaches. The Logit-Only GARP approach does allow for use of
interaction variables and categorical variables where the superset does not, making it an interesting
approach. The GARP superset did overpredict compared to the logit-only model with comparable
variables (Figure 3). More work is needed to explore the differences in rulesets to understand how these
models differed. The Random Forest approach does allow for a direct measure of variable contribution,
adding value to understanding the role of variables in the predictions (Figure 4). This is especially
important when trying to understand the role of variables for host versus the pathogen.
Chapter 4: Measuring inter-annual dynamics of Low-land Plague Focus
in Azerbaijan using historical maps and STAMP
Introduction
Plague, caused by Yersinia pestis, is a bacterial zoonosis with enzootic cycles in rodents and small
mammals. In Azerbaijan, the Trans-Caucasian Lowland-Foothills focus spans much of the arid plains
across the central portion of the country. In this region, the Libyan Gird, Meriones libycus, is the primary
mammalian reservoir for plague. Enzootic transmission cycles in most of the world involve interactions
between partially resistant rodents and fleas. Historically, annual surveillance for Y. pestis was
associated with zoological expeditions across known colonies of M. libycus or other mammial host
species within the Azerbaijani plague foci. As part of these surveillance efforts, the Republican AntiPlague Station collated annual sampling data and produced accurate, hand-drawn maps delineating
areas of gird abundance based on five categories (very low, low, average, high, and very high) (Figure 1).
Each category is associated with a defined population and mammal density range. Annual maps from
1972 to 1985 (excluding 1984) were published in annual yearbooks summarizing surveillance activities.
The maps were heads-up digitized within a geographic information system (GIS) to evaluate spatial and
temporal patterns of density for M. libycus. These annual range maps can be used to understand the
historical distribution and persistence of M. libycus.
The objectives of this study were:
•
to employ a Space Time Analysis of Moving Polygons (STAMP)
approach in an effort to understand how rodent abundance changes
over space and time
•
to identify areas of spatially and temporally stable rodent abundance
Materials and Methods
Database development
We photographed all maps from all yearbooks between 1972 and 1985; minus 1984 (we could not
locate this book in the archive). All photographs were captured as digital *.jpg images and organized
by year. A small Olympus digital camera was used to capture high resolution photos. Each yearbook
was opened map page-by-map page and photographed two times (for quality comparisons). The
digital camera was held near perpendicular to the map image laid out flat on a table (Figure 1). In
total, 184 maps were photographed from the 13 year period. All maps were cataloged according to
translated map titles and legends. All map images were geographically referenced using the
georeferencing tools in ArcGIS v 9.3.1. All maps were referenced back to a single map and shapefile
of Azerbaijan to reduce error or distortion in georeferencing. All yearbooks used a single basemap of
Azerbaijan across years, reducing overall error in referencing. For this study, w selected out M.
libycus records for each year and constructed data layers for spatial analysis.
STAMP
STAMP is a tool that measures and quantifies change across consecutive time periods. STAMP
intersects area polygons from two time periods, and new polygons are formed from the
intersection. The new polygon layers are assigned an event type defined by the topological
relationship between the two time periods. There are five potential event categories: expansion,
contraction, stable, disappearance, generation (Figure 1). Events created from spatial overlap can
be defined as contraction, expansion, or stable. Disappearance and generation events are spatially
isolated. Contraction and disappearance are areas found exclusively in time period one. Generation
and expansion are found exclusively in time period two. Stable is an area common to both time
periods.
A STAMP analysis was used to explore the spatial changes of M. libycus abundance across the
landscape from 1972 to 1985. A separate STAMP analysis was conducted on each of the five
abundance categories. Each STAMP analysis included polygons from every year that data was
available for each abundance category. There was no data available on M. libycus abundance for
1979, or 1984 in any abundance category. Every year from 1972 to 1985 included M. libycus
abundance for low, average, and high abundance categories. Very low abundance data was available
from 1972 to 1978. Very high abundance was available for every year except 1973, 1976, and 1982.
The area of each abundance category for each year was also calculated.
Stable Clusters
Stable clusters were identified by extracting stable event polygons from STAMP outputs for each
abundance category. Overlapping stable layers from consecutive years within each abundance
category were identified. The overlapping portion of the polygon was used to create clusters of
stability. Only clusters spanning a time period greater than two years were of interest for this
analysis.
Discussion
The goal of this study was to understand changes in M. libycus abundance in Azerbaijan from 1972 –
1985. We were particularly interested in identifying regions of long term stability in rodent
abundance. Future disease surveillance will be benefitted by an understanding of historical
persistence and variability of host species across the landscape.
We found that there was variability in the spatial distribution of M. libycus over the 13 year period in
all abundance categories. Regions characterized by low M. libycus abundance were the most stable
over time with several regions persisting for more than five years. For the majority of abundance
categories, regions of stability did not persist for more than five years. Ongoing surveillance is
important in order to keep up with the changes in M. libycus distribution. Every abundance
category had a stable region that was maintained for at least two years.
Since the collapse of the Soviet Union, annual surveillance efforts have been limited across
Azerbaijan. Analyzing the characteristics associated with these stable regions will provide more
insight into the habitat preferences of M. libycus. In the absence of extensive surveillance, sampling
efforts could focus on stable areas across categories to target known historical areas of persistence
populations of mammals (average categories) or those areas of high abundance. These analyses
provide a baseline for guiding modern surveillance for this important pathogen and host.
Figure 1. Historical plague reports, The maps in the left panel and right panel illustrate the types of
maps in the annual plague yearbooks (shown center panel). All maps were laid flat, photographed,
digitized in a GIS and converted to polygon files of seasonal abudance categorized from very low to
very high. These data were then used to construct spatio-temporal analyses using the STAMP package
in ArcGIS 9.3.1 (http://www.geog.uvic.ca/spar/stamp/help/index.html)
Figure 2. STAMP event definitions, each circle represents an area in one of two consecutive time
periods. Concentration, stable, and expansion are defined by overlapping areas, but contraction is
only in time period one while expansion is only in time period two. Disappearance and generation are
events that are spatially isolated from other areas.
Figure 3. Very low abundance STAMP output. This map shows STAMP classifications for changes in
very low abundance between 1976 and 1977.
Figure 4. Low abundance STAMP output. This map shows STAMP classifications for changes in low
abundance between 1976 and 1977.
Figure 5. Average abundance STAMP output. This map shows STAMP classifications for changes in
average abundance between 1976 and 1977.
Figure 6. High abundance STAMP output. This map shows STAMP classifications for changes in high
abundance between 1976 and 1977.
Figure 7. Very high abundance STAMP output. This map shows STAMP classifications for changes in
very high abundance between 1976 and 1977.
Figure 8. Very low abundance stable clusters. This map shows the areas of very low M. libycus
abundance that remain stable for 2 or more consecutive years.
Figure 9. Low abundance stable clusters. This map shows the areas of low M. libycus abundance that
remain stable for 2 or more consecutive years.
Figure 10. Low abundance stable clusters. This map shows the areas of low M. libycus abundance
that remain stable for 5 or more consecutive years.
Figure 11. Average abundance stable clusters. This map shows the areas of avearge M. libycus
abundance that remain stable for 2 or more consecutive years.
Figure 12. High abundance stable clusters. This map shows the areas of high M. libycus abundance
that remain stable for 2 or more consecutive years.
Figure 13. Very high abundance stable clusters. This map shows the areas of very high M. libycus
abundance that remain stable for 2 or more consecutive years.
Table 1. Changes in abundance area over time. The total area for each abundance category for each
year is reported.
Chapter 5: Mapping human tularemia in Azerbaijan using historical data
Figure 1. Rayon-level map of human tularemia cases in Azerbaijan from 1958-2001 based on APS
records. Green dots represent Francisella tularensis isolates from the APS pathogen passport database
constructed during the AJ-3 project.
Chapter 6: Spatial patterns of livestock brucellosis in Azerbaijan 2002 to
2010
Introduction
Brucellosis is the most common bacterial zoonosis worldwide. Independent nations of the
former Soviet Union have been disproportionately burdened by some of the highest rates of the disease
in both livestock and humans (Pappas and others 2006). In Azerbaijan brucellosis is endemic in livestock
and is responsible for significant economic losses as well as representing a substantial burden to public
health. This study had two main objectives: 1) determine the prevalence of brucellosis in large
(cattle/bovine) and small ruminants (sheep/ovine and goats/caprine) and 2) identify significant hotspots
of brucellosis.
Materials and Methods
During the period from 2002 - 2010, a team from the State Veterinary Service (SVS) in Baku
conducted livestock sero-surveillance for brucellosis across all districts of Azerbaijan. Sera were
collected from a total of 11,740,974 large (cattle) and 5,028,599 small ruminants (sheep). Serology was
conducted using the Rose Bengal Test (RBT) to detect antibodies against Brucella spp. The study analysis
was grouped into 3-three year periods: Period 1 (2002 to 2004), Period 2 (2005 to 207), and Period 3
(2008 to 2010). Apparent prevalence (AP) estimates were calculated as a percent for large and small
ruminants separately using the ratio of test positives to the total number of animals sampled in a given
period. Bayesian true prevalence (TP) estimates were derived using WinBugs to account for the
imperfect sensitivity and specificity of the RBT. Prior distributions were constructed using beta buster
(UC Davis , http://www.epi.ucdavis.edu/diagnostictests/betabuster.html) for sensitivity with a mode of
0.75 (95% CI: 0.60) and specificity with a mode of 0.85 (95% CI: 0.75). Models were run using 100,000
iterations with a burn in of 20,000 and applying the following WinBugs code:
model
{for (i in 1:k)
{
y[i] ~ dbin(ap[i], n[i])
ap[i] < -tp[i] * Se + (1 - tp[i]) * (1 - Sp)
tp[i] ~ dbeta(1, 1)
}
Se ~ dbeta(23.56, 8.5)
Sp ~ dbeta(46.35, 9) }
### Initial Values ###
list(tp=c(0.5), Se = 0.5, Sp = 0.5)
Spatial Mapping
Local cluster analysis was performed at the district level using the Local Moran’s I statistic [1] in
the GeoDa software package [2] using the period prevalence estimates during each of the three time
periods for both cattle and sheep separately. The statistic can identify hotspots as well as spatial
outliers, or in this case individual districts, that vary disproportionately from the global mean. Districts
are deemed to be not significant or a cluster of either High-High ( i.e. high values of brucellosis
prevalence surrounded by high values), Low-Low, High-Low, or Low-High values relative to neighboring
rayons at a given probability level p < 0.05 using 999 randomizations. The null hypothesis states that
there is no spatial autocorrelation or association of human brucellosis cases between districts. The local
Moran’s I statistic is written following [1]:
Ii Zi Wij Zj
j
Results
Figure 1 shows boxplots of AP and TP prevalence the estimates for all three time periods across
all districts in Azerbaijan. Boxplots illustrate the range of estimates and show significant a general
pattern of an over all high median TP. Results from the mapping in Azerbaijan showed spatial variability
in the distribution of disease. Large ruminant prevalence estimates at the district level ranged from a
low of 0% (95%CI: 0, 0.03) to a high of 4.1% (95%CI: 0.3, 14.5) in Period 1, 1.8 % (95%CI: 1.5, 2.1) in
Period 2, and 51.2% (95%CI: 50.6, 51.7) in period 3 (Figure 2). Small ruminants prevalence estimates at
the district level ranged from a low of 0% (95%CI: 0, 0.03) to a high of 6.7% (95%CI: 4.6, 9.7) in Period 1,
7.6 % (95%CI: 0.2, 35.4) in Period 2, and 10.3% (95%CI: 18.3, 41.6) in period 3 (Figure 3). Figure 2A-2C
and 3A-3C show the results from the Local Moran's I analysis that identified hotspot clusters (i.e. high
values surrounded by high values) around Baku for both small and large ruminants in periods 1 and 2.
During period 3 spatial outlier clusters representing High-Low (i.e. high areas surrounded by low areas)
districts were identified in the southwestern part of the country for sheep (Figure 3C) and in the
southeast for cattle (Figure 2C).
Discussion
Brucellosis is major concern for both humans and livestock in Azerbaijan. Cuts in the funding
for veterinary and public health management have highlighted the importance of continued disease
surveillance in order to better implement control strategies. The analyses here looked at nine years of
nationwide sero-surveillance in both large and small ruminants. The findings indicate the presence of
hotspots of livestock brucellosis. These hotspots may represent possible foci for the disease in both
animals and humans. A recent study by the Anti-Plague station in Baku [3] identified a High-Low cluster
(a district with a value surrounded by districts with low values) of human brucellosis during the time
period 2005-2009 in the Yardymil district (southeast Azerbaijan), which corresponds to a High-Low
cluster of cattle brucellosis identified in this study during period 3 in the same district. In both studies
the cluster outlier emerged later in the study period, which potentially a change in the disease status
and the emergence of a new focus of the disease.
In this study the number of herds sampled was unviable and represents a potential source of
bias in determining the sero status of livestock throughout the country. Additionally, sampling strategies
may have changed over time to target specific areas with ongoing brucellosis transmission. Overall the
analyses presented here may allow for a better evaluation of the TP of livestock brucellosis and help
direct future research or surveillance efforts.
References
1. Anselin L (1995) Local indicators of spatial association—LISA. Geographical Analysis 27: 93-115.
2. Anselin L, Syabri I, Kho Y (2006) GeoDa: An introduction to spatial data analysis. Geographical Analysis
38: 5-22.
3. Abdullayev R, Kracalik IT, Ismayilova R, Ustun N, Talibzade A, et al. (2012) Analyzing the spatial and
temporal distribution of human brucellosis in Azerbaijan (1995-2009) using spatial and spatio-temporal
statistics. BMC Infectious Diseases 12: 185.
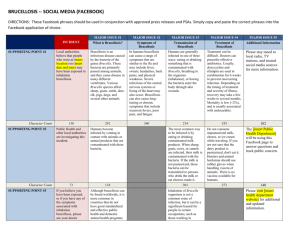
Figure1. Boxplots are displaying the apparent prevalence (AP) (%) and Bayesian true prevalence (TP)
(%) estimates per district in each of the time periods, Period 1 (2002 to 2004), Period 2 (2005 to 207),
and Period 3 (2008 to 2010). AP estimates were derived using the ratio of Rose Bengal test positives
to the total number of animals sampled in each period. TP estimates were derived following the
procedure outlined in the methods.
Figure 2. Brucellosis apparent prevalence (ap) estimates (%) calculated from sero-surveillance in cattle
and grouped into three equal 3-year time periods. The map displays Period 1 (2002 to 2004), Period 2
(2005 to 207), and Period 3 (2008 to 2010). Insets 1 refers to Period1, inset 2 to Period 2, and inset 3 to
period 3. Insets A, B, and C refer to clustering results from periods 1,2,and 3, respectively. Local
Moran’s I clusters across Azerbaijan are shown with red portraying High-High areas , and pink HighLow areas.
Figure 3. Brucellosis apparent prevalence (ap) estimates (%) calculated from sero-surveillance in
sheepand grouped into three equal 3-year time periods. The map displays Period 1 (2002 to 2004),
Period 2 (2005 to 207), and Period 3 (2008 to 2010). Insets 1 refers to Period1, inset 2 to Period 2, and
inset 3 to period 3. Insets A, B, and C refer to clustering results from periods 1,2,and 3, respectively.
Local Moran’s I clusters across Azerbaijan are shown with red portraying High-High areas , and pink
High-Low areas.
Table 1. National estimates of brucellosis prevalence by livestock type and year. Test prevalence per
1,000 animals was calculated using the ratio of test positives to the total numbered sampled.
Chapter 7: The status of zoonoses in Azerbaijan during Soviet and postSoviet governance: Analyzing space-time patterns of human brucellosis
and anthrax
Introduction
In Azerbaijan the collapse of the Soviet Union has brought about major changes to the livestock sector.
The breakdown of collective and state farms led to widespread privatization, resulting in diminished
livestock control measures, which has further contributed to the occurrence of human zoonoses.
Analyzing the spatial patterns of health events may offer insight into the etiology of a disease and
provide crucial information on any potential changes in the distribution of a disease (Jacquez 1996). The
purpose of this study was to examine space-time patterns of incident human disease during Soviet and
post-Soviet governance to study the possible re-emergence of two major livestock zoonoses, anthrax (
Bacillus anthracis) and brucellosis (Brucella spp.).
Materials and Methods
A database of disease records consisting of case counts and incident occurrences of human disease per
rayon were derived from the archival records at the Anti-Plague Station in Baku, Azerbaijan. This
database was used to analyze the data spatially while comparing human anthrax and brucellosis records
from Soviet (1983 to 1991) and post-Soviet (1992 to 2000) governance.
Linear trend analysis was used identify whether there was an increasing or decreasing trend in the
incidence of anthrax and brucellosis. The bivariate local Moran’s I (Anselin 1995) was employed to
identify patterns of incident disease (anthrax or brucellosis) for each rayon by comparing rates spatially
between Soviet and post-Soviet governance. This test will identify whether or not high or low incident
rates during the Soviet period clustered around high or low incident rates from the post-Soviet period.
Additionally, SaTScan was used to test for spatial variation in a temporal trend by identifying individuals
rayons that may be contributing to a decreasing or increasing trend in the disease rate.
Results
The results show there were 175 cases of human anthrax and 1,822 cases of brucellosis during the
Soviet Period 1983 to 1991 and 250 cases of anthrax and 5,731 cases of brucellosis during the postSoviet period 1992 to 2000. (Figure 1-6).Linear trend analysis indicated that there was a significant
increase in the incidence of brucellosis over time R2 = 0.77 ( Figure 1,7) however there was no significant
trend found for anthrax incidence over time R2 = 0.00053 (Figure 2,7). The bivariate local Moran’s I
showed that there was persistence in the occurrence of high incidence for both anthrax and brucellosis
across time periods with high clustering rayons shown in red (Figure 8). The SaTScan analysis revealed
the presence of local fluctuations in the disease rate across time. Figure 9 shows there was a 12.5%
percent annual increase in anthrax inside the buffer compared to a 2.9% decrease outside and a 18.4%
percent annual increase in brucellosis inside the buffer compared to a 8.9% increase outside the buffer.
Discussion
The analysis of human anthrax and brucellosis illustrate the potential for a changing baseline occurrence
of disease. Political and economic changes represent a threat to public and veterinary health. The
increased rates of disease following the collapse of the Soviet Union highlight the need for sustained
livestock management as well as the potential for the emergence of new or current infectious diseases if
current health policies are not successful.
References
Anselin, Luc. 1995. Local indicators of spatial autocorrelation. Geographical Analysis. 27:93-115.
Anselin, Luc, I. Syabri, and Y. Kho. 2006. GeoDa: An introduction to spatial data analysis. Geographical
Analysis. 38:5–22.
Jacquez, Geoffrey M. 1996. A k nearest neighbor test for space-time interaction. Statistics in Medicine.
15:1935-1949.
800
120
700
100
600
R² = 0.7726
80
Cases
Incidence
500
400
60
300
40
200
20
100
0
0
1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000
Year
Figure 1. Yearly incidence per 1,000,000 population and case totals of human brucellosis in Azerbaijan
during the period 1983 to 2001. Grey bars show case totals during the period of Soviet governance and
black bars show the period post-Soviet governance. The dotted red line shows the incidence per year
and the blue line describes the linear trend over time R2 = 0.77.
80
14
70
Incidence
12
60
Linear
R² = 0.0003
(Incidence)
10
Incidence
50
CASES
8
40
6
30
4
20
2
10
0
0
1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000
YEAR
Figure 2. Yearly incidence per 1,000,000 population and case totals of human anthrax in Azerbaijan
during the period 1983 to 2001. Grey bars show case totals during the period of Soviet governance and
black bars show the period post-Soviet governance. The dotted red line shows the incidence per year
and the blue line describes the linear trend over time R2 = 0.00053.
Figure 3. Map shows human brucellosis case totals by rayon during the period of Soviet governance
1983 to 1991.
Figure 4. Map shows human brucellosis case totals by rayon during the period of post-Soviet
governance 1992 to 2000.
Figure 5. Map shows human anthrax case totals by rayon during the period of Soviet governance 1983
to 1991.
Figure 6. Map shows human anthrax case totals by rayon during the period of post-Soviet governance
1992 to 2000.
Figure 7. Map shows the average incident reporting per 100,000 population for human anthrax and
brucellosis during Soviet and post-Soviet governance. Map A1 shows the average anthrax incidence by
rayon during the period 1983 to 1991 and map A2 shows the average anthrax incidence during the
period 1992 to 2000. Map B1 average brucellosis incidence by rayon during the period 1983 to 1991
and map A2 shows the average brucellosis incidence during the period 1992 to 2000
Figure 8. Map shows the results of the bivariate local Moran’s I. The top map shows clustering between
periods for anthrax and the bottom map shows clustering between periods for brucellosis. Rayons in red
show clustering of high values between Soviet and post-Soviet eras. Areas in dark blue show clustering
low values and areas in light blue show clustering of low values during the Soviet period around High
values during the post Soviet period.
Figure 9. The top map displays a 12.5% percent annual increase in anthrax inside the buffer compared to
a 2.9% decrease outside. The bottom map displays a 18.4% percent annual increase in brucellosis inside
the buffer compared to a 8.9% increase outside.
Chapter 8: Analyzing the spatial and temporal distribution of human
brucellosis in Azerbaijan (1995 - 2009) using spatial and spatiotemporal statistics
This work was published in BMC Infectious Diseases in 2012.
Abstract
Background: Human brucellosis represents a significant burden to public and veterinary health globally,
including the republic of Azerbaijan. The purpose of this study was to examine and describe the spatial
and temporal aspects of the epidemiology of human brucellosis in Azerbaijan from 1995 to 2009.
Methods: A Geographic information system (GIS) was used to identify potential changes in the spatial
and temporal distribution of human brucellosis in Azerbaijan during the study period. Epidemiological
information on the age, gender, date, and location of incident cases were obtained from disease
registries housed at the Republican Anti-Plague station in Baku. Cumulative incidences per 100,000
population were calculated at the district level for three, 5-year periods. Spatial and temporal cluster
analyses were performed using the Local Moran's I and the Ederer-Myer-Mantel (EMM) test.
Results: A total of 7,983 cases of human brucellosis were reported during the 15-year study period.
Statistically significant spatial clusters were identified in each of three, five year time periods with
cumulative incidence rates ranging from 101.1 (95% CI: 82.8, 124.3) to 203.0 (95% CI; 176.4, 234.8).
Spatial clustering was predominant in the west early in the study during period 1 and then in the east
during periods 2 and 3. The EMM test identified a greater number of statistically significant temporal
clusters in period 1 (1995 to 1999).
Conclusion: These results suggest that human brucellosis persisted annually in Azerbaijan across the
study period. The current situation necessitates the development of appropriate surveillance aimed at
improving control and mitigation strategies in order to help alleviate the current burden of disease on
the population. Areas of concern identified as clusters by the spatial-temporal statistical analyses can
provide a starting point for implementing targeted intervention efforts.
Keywords: brucellosis; spatio-temporal analysis; Azerbaijan; former Soviet Union; serology
Introduction
Brucellosis is a widespread zoonotic disease regarded as an emerging and re-emerging threat to
public and veterinary health worldwide [1]. Developing nations are often disproportionately afflicted
resulting in significant economic losses while at the same time exacting a heavy toll on the health of
populations [2, 3]. Regions most heavily burdened by the disease include countries of the
Mediterranean, Central Asia, Middle East, Latin America, Sub-Saharan African and Balkan Peninsula [4].
The causative agents of the disease are a group of pathogenic bacteria in the genus Brucella, which
primarily infect animal reservoirs. The primary agents of infection in humans are B. abortus (cattle), B.
melitensis (sheep and goats), B. suis (swine), and B. canis (dogs) [5]. Humans are often secondarily
infected through the consumption of unpasteurized dairy products or coming into contact with infected
material during animal husbandry or meat processing [1].
Clinical signs and symptoms of the disease are usually acute and nonspecific, often mimicking
other illnesses [6]. Common reported symptoms include fever, malaise, fatigue, sweats, chills, weight
loss, and myalgia [7, 8]. In some circumstances brucellosis infections can develop into a chronic form,
which consists of the continuation of symptoms for greater than twelve months after a diagnosis [7, 9].
Despite the reduction or elimination of the disease in many countries through vaccination efforts or
increased food safety standards it is estimated that there are nearly 500,000 new cases of the disease
each year worldwide [10].
The disease was first diagnosed in Azerbaijan in 1922 and quickly established itself, spreading to
more than two thirds of the country’s districts in less than thirty years [11]. Recent governmental
changes brought on by the collapse of the Soviet Union in 1991 have likely contributed the persistence
of the disease during the last two decades, due to decreased funding for surveillance and eradication
programs [4, 12]. Out of the sixty two countries identified as having the highest national incidence of
brucellosis, Azerbaijan currently ranks thirteenth with an estimated annual incidence through the year
2000 at over 50 cases per million [4].
Despite the current status of human brucellosis in Azerbaijan, there have been no known
published efforts to map and describe the occurrence of the disease at a local level. In order to better
understand the spatial and temporal distribution of human brucellosis in Azerbaijan, geospatial
analytical techniques and a geographic information system (GIS) were employed. Previous studies using
spatio-temporal methods to investigate the distribution of health events have been successful in
uncovering factors related to the occurrence of disease [13, 14]. . Research on brucellosis incorporating
spatial analyses identified areas of high human case reporting associated with specific ethnic
populations and the consumption of unpasteurized food products in California [6] and Germany [15].
There were three primary objectives of this study: 1) to describe the spatial and temporal
distribution of human brucellosis in Azerbaijan; 2) identify the potential presence of clusters of the
disease in space and time; and 3) identify epidemiological characteristics of individuals that were
diagnosed with an infection.
Methods
Ethics Statement
No human subjects work was undertaken in this study, human brucellosis case data were extracted from
annual government reports. These government reports are prepared public reports, providing
summarized count data of patients diagnosed at government health care facilities by category of disease
and year. All data were anonymised.
Data Collection and Management
Brucellosis is a nationally reportable infectious disease in Azerbaijan. Surveillance and documentation of
health events with in the country are undertaken by their surveillance and diagnostic laboratory known
as the Anti-Plague Station (APS), which is divided into five reporting zones. Each of the five reporting
zones has a Regional APS (RAPS) office that responds to health inquiries in order to obtain laboratory
samples and verify any diagnosis. In this study a case was defined as any individual with a confirmed
positive serology test for Brucella spp. Suspected cases of human brucellosis are confirmed by
laboratory testing with the Rose Bengal, Huddleson, and the Wright serum agglutination tests. Initial
laboratory tests are performed at RAPS, with confirmation (through repeat tests) at the Republican APS.
The reporting district was assumed to be the origin of the infection for the individual and the
locations of human brucellosis seropositives were aggregated to the 66 districts in Azerbaijan [6]. In
order to analyze and describe the spatial and temporal distribution of the disease the 15-year study
period was grouped into three, 5-year periods with the total number of new cases per period
aggregated to the district level as follows: period 1 (1995 to 1999), period 2 (2000 to 2004), and period 3
(2005 to 2009). Cumulative incidences were calculated per district for each of the three, 5-year periods
with the total number of cases during each period as the numerator and the median year population of
each 5-year period as the denominator. Population estimates for incidence rates were obtained from
the RAPS and the Azeri State Statistical Committee (http://www.azstat.org/). Yearly case totals and the
annual incidence of human cases per 100,000 population in Azerbaijan were recorded during the 15year study period (Figure 1). Epidemiological data on the age and sex of seropositive cases were
obtained from disease registries and stratified by male and female sexes into the following age groups:
0-7, 8-11, 12-15, 16-19, 20-29, 30-39, 40-49, 50-59, and >60 years. Azerbaijan was divided into the
dummy regions of Nakhchivan, West, Central, and East in order to better describe the spatial and
temporal distribution of the disease (Figure 2). It was not possible to match individuals along with their
age and gender back to the district level therefore not used in the spatial analytical component of this
study. Since brucellosis infections can have symptoms that mimic other diseases and may have an
unknown date of onset the date the infection was diagnosed was used included in this analysis rather
than the date of the onset of symptoms [6].
Spatial Analysis
Cumulative incidences per 100,000 individuals were mapped at the district level for each of the
three 5-year time periods to highlight any spatial changes in risk over time. A second administrative
level (district) shapefile of Azerbaijan was obtained from the Global Amidnistrative Areas database
(www.gadm.org). Smoothed risk estimates were calculated from crude cumulative estimates for each
time period using the Empirical Bayesian Smoother (EBS) in the GeoDa software package [16], with the
total number of cases in each period as the numerator and the median year population of each period
as the denominator. The EBS technique can be used to adjust for instability in the risk estimates caused
by heterogeneity in the distribution of cases and the population. It has been suggested that the EBS
methodology can be implemented in several scenarios such as when the numerator data total less than
three cases, which was the situation in this analysis [17]. In order to maintain a standard comparison of
rates between time periods as well as crude and EBS estimates cumulative incidences were choropleth
mapped in the following categories per 100,000 persons 0, 0 to 30, >30 to 60, >60 to 90, and >90. All
maps were produced in ArcGIS 9.3.1 (ESRI, Redlands, California). Cumulative rates for EBS and crude
estimates were compared by dummy region using box plots created in SAS v9.2.
Spatial Autocorrelation
Local cluster analysis was performed using the Local Moran’s I statistic [18], a local indicator of
spatial autocorrelation (LISA), in the GeoDa software package [16] using the cumulative incidence rates
at the district level in each period as the variable of interest. The statistic can identify hotspots as well
as spatial outliers, or in this case individual districts that vary disproportionately from the global mean.
Districts are deemed to be not significant or a cluster of either High-High, Low-Low, High-Low, or LowHigh values relative to neighboring districts at a given probability level p < 0.05 using 999
randomizations. The null hypothesis states that there is no spatial autocorrelation or association of
human brucellosis cases between districts. The local Moran’s I statistic is written following [18]:
Ii Zi Wij Zj
j
Where Ii is the statistic for a district I, Zi is the difference between the brucellosis risk at I and the mean
brucellosis for Azerbaijan, Zj is the difference between brucellosis risk at j and the mean for Azerbaijan.
Wij is the weights matrix that in this case only considers neighbors that share a common border or
vertex (in the Queen contiguity case Wij is 1/n if a district shares a border or a vertex and zero
otherwise).
Temporal Analysis
Information on the month of diagnosis of the reported cases was examined to identify the
presence of a possible seasonality in the reporting of cases. Monthly cases were aggregated into
seasons, defined as winter (December, January, February), spring (March, April, May), summer (June,
July, August), and fall (September, October, November). Additionally, the Ederer-Myer-Mantel (EMM)
test [19] was implemented in ClusterSeer2 [20] in order to explore the presence of temporal clusters of
human brucellosis cases by districts in Azerbaijan during the 15-year study period. This test uses the
total number of cases per district within a consecutive time period and identifies observations that
deviate from an expected number of cases and is written following [19]:
χ2 =
[|∑m1 − E(∑m1 )| − 0.5]2
∑Var(m1 )
where m is the greatest case total for human brucellosis occurring in a district in any of the three time
intervals of time series i. Since the statistic is biased to changes in population over time [6, 19],
consecutive time series were limited to 5-year totals resulting in the analysis of three 5-year intervals
during the study period. Each of three time intervals for the same district are treated as independent
observations in this analysis [6].
Results
Spatial Analyses
During the study period 1995 to 2009 there were 7,983 reported cases of human brucellosis in
Azerbaijan (Figure 1). Annual case totals ranged from 756 in the year 1996 to 392 cases in the year 2009
with a median number of reported cases per year of approximately 522.5 (95% CI: 429, 591). A high
percentage of areas reported persistence of disease; out of the 66 districts in the country 65% reported
having at least one case of human brucellosis every year during the 15-year study period (Figure 2). The
number of districts reporting at least one case for every year in the study also showed regional
differences: 1 (16.7%) district in Nakhchivan, 12 (63%) districts in the West, 16 (84%) districts in the
Central, and 14 (61%) districts in the East.
Crude and Empirical Bayes Smoothed (EBS) cumulative incidence maps indicated the presence
of spatial variation in the distribution of risk across Azerbaijan during the three, 5-year time periods
(Figure 3). During period 1 cumulative incidences ranged between 0 to 415.0 cases per 100,000 persons
with the highest rate occurring in Bilasuvar (95%CI : 368.9, 465.4) within the Central region. Period 2
displayed cumulative incidences that ranged between 0 to 386.5 cases per 100,000 persons with the
highest rate occurring in Gobustan (95%CI : 324.5, 456.9) within the Eastern region. Cumulative rates
during period 3 ranged between 0 to 283.4 cases per 100,000 persons with the highest incidence again
occurring in Gobustan (95%CI : 232.2, 342.8). EBS cumulative incidence maps illustrated minor variation
in the distribution of risk, indicating relative stability in the calculated spatial estimates (Figure 3 see B
insets)). Boxplots of cumulative rates stratified by time period and method of estimation are shown by
region in Figure 4. The distribution of cumulative rates indicated that there was less than 5% variability
between the crude and smoothed estimates within regions during each time period.
Spatial Autocorrelation
The LISA cluster analyses identified significantly high-high clusters of human brucellosis cases at
the district level during each of the three periods indicating districts of high incidence surrounded by
other districts of high incidence (Figure 5). During period 1 high-high clusters were identified in the
west while clusters were found in the east during periods 2 and 3. A total of five high clusters were
identified during period 1 that included the districts of Goranboy , Imishli, Khanlar, Saatly, and Tatar with
corresponding cumulative rates per 100,000 persons: 203.0 (95% CI; 176.4, 234.8), 101.1 (95% CI: 82.8,
124.3), 116.1 (95% CI: 88.4, 151.9), and 74.2 (95% CI: 55.8, 95.0) respectively. A High-High cluster
identified during period 2 included Absheron with 41.1 (95% CI: 28.7, 57.2) cases per 100,000 persons.
The LISA analysis for period 3 identified Khizi as a high-high cluster with 36.0 (95% CI: 11.7, 84.0) cases
per 100,000 persons. Areas of Low-Low clustering identified by the analyses illustrated an absence or
low levels of disease risk among districts in southwestern Azerbaijan that persisted during each time
period (Figure 5). A similar pattern held true for the southeastern tip of Azerbaijan with the exception
of Yardymli during period 3, which was identified as High-Low cluster indicating that it had a high
cumulative rate compared to neighboring districts.
Temporal analyses
There appears to be a distinct seasonality in the temporal distribution of monthly human
brucellosis cases (Figure 6). Reporting of cases was highest in the month of July with a total of 1045
cases (13.1%) during the fifteen year period. The Summer season accounted for the greatest number of
cases 3,131 (39.2% ) followed by Spring 2,228 (27.9% ) then Fall 1,624 (20.3% ) and lastly Winter with
1,000 cases (12.5% ). Results from the EMM test identified 29 districts that were part of statistically
significant temporal clusters (Figure 7). There were a greater number of temporal clusters that occurred
earlier in the study period; period 1 (n=16) (χ2 = 102.3, p < 0.001), followed by period 2 (n=9) (χ2 = 29.5,
p<0.001), and period 3 (n=4) (χ2 = 19.2, p<0.01). Temporal clusters from period 1 were located
predominantly in the Central and Western regions of Azerbaijan while clusters identified during period 2
and 3 were more evenly dispersed across the country; however, there were no statistically significant
human brucellosis clusters identified in the Nakhchivan region.
Age and Gender
Age and sex stratified case totals illustrated a disproportionate number of cases reported among
males, with males more heavily burdened by the disease across all age groups (Figure 8). There were a
total of 5,730 cases in males accounting for approximately 71.8% of all reported infections. Among age
groups for both sexes, 16 to 19 year olds accounted for 2,230 cases [27.9% (95% CI: 27.7, 28.2)] while 20
to 29 year olds accounted for 3,431 cases [43.0% (95% CI: 42.6, 43.3)], with a combined total of 5,661
cases [70.9% (95% CI: 70.6, 71.2)] respectively.
Discussion
This study represents the first attempts to map and describe local (district level) patterns of
human brucellosis in Azerbaijan. We used GIS in conjunction with spatial and temporal analytical
methods to analyze patterns of cumulative incidence at the district level in Azerbaijan finding evidence
of clustering in both space and time. Additionally, our analyzes confirm that human brucellosis persists
annually throughout much of the country, particularly the central region. These current analyses affirm
the notion that brucellosis has become endemically established since the first documented reporting in
the 1920s.
The results of this study indicated variation in the spatial and temporal distribution of brucellosis
reporting in Azerbaijan. Spatial clusters of cumulative incidence were identified in each time period
indicating localized areas of high case reporting with clusters predominant in the west early in the study
during period 1 and then in the east during periods 2 and 3. Temporal clustering was higher during
period 1, but persisted across periods 2 and 3. The median reporting of cases during the study period
exceeded 500 cases annually with approximately 65% of districts reporting at least one case every year
during the study period, suggesting the possibility of local (district level) persistence. The number of
reported cases most likely represents a significant underestimation of the actual disease burden across
the country as human brucellosis is often misdiagnosed or is unreported [21]. Additionally, traditional
serological tests, like Rose Bengal can have relatively low sensitivity [22]. In Azerbaijan, these issues are
likely confounded by the underutilization of the nation's healthcare facilities [23].
The temporal distribution of cases showed that there was a greater proportion of brucellosis
reporting during period 1(1995 to 1999). Temporal clusters identified by the EMM test also indicated a
greater number of significant temporal clusters during period 1, which further corroborated that a larger
proportion of cases occurring earlier in the study period. Transformations in the funding and
organization of public health management and surveillance during the transitional phase to
independence have likely contributed to the elevated reporting of the disease in Azerbaijan during this
period [23]. However, temporal clustering also suggests that the disease persisted through the more
contemporary time period (period 3). The clusters in this most recent period reflect new areas of
increased cases reporting that may not be directly attributed to spillover from the Soviet period. This
highlights the need for continued surveillance, particularly in those districts identified.
The seasonality of brucellosis can often be attributed to the seasonal birthing of small ruminants
(goats and sheep) [24, 25]. A recent study in Greece indicated that human cases were directly related to
the parturition of small ruminants [26]. In Azerbaijan the birthing of small ruminants occurs in early
spring and is often demarcated by an increase in the incidence of livestock brucellosis [11]. Reporting of
brucellosis in this study shows a concordance with the spring/summer birthing of small ruminants, with
a greater number of cases reported during the summer and spring months (March - August) with a peak
in reporting in July. Similar findings related to the seasonality of the disease were also reported in
Uzbekistan and Italy [27, 28].
As brucellosis is not a contagious disease, spatial clusters of human cases are most likely a result
of shared food sources, animal processing, more intensive agricultural production zones, or similar
socio-cultural practices [6]. Clusters of disease in this case are also most likely indicative of a larger
underlying prevalence of the disease in the local livestock population. High-High districts identified by
the LISA analysis during the three time periods indicated a potential shift in the pattern of high case
reporting as clusters of the disease present in the west during period 1were absent in periods 2 and 3.
Spatial differences in the reporting of brucellosis over time may be a result of changes in agricultural
production, with steep declines in production noted during the period 1991 through 1996 followed by
efforts to gradually restore sustained agricultural output [29]. These changes have resulted in the
subsequent spatial restructuring of agricultural production brought on by the abandonment of
government controlled collective farms and animal processing facilities post-collapse of the Soviet Union
[30]. Decollectivization marked a shift towards privatization of animals and farms beginning in 1996 [31]
resulting in decreased control of veterinary health management policies [22, 32]. This has consequently
allowed for eased restrictions on the location of agricultural operations as well as trans-boundary
movements and production of animals throughout the country [11]. The move towards a more
privatized market has fueled expansion around larger cities particularly in the east around the capital
Baku [31]. Additionally, the conflict with Armenia, which shares a border with the west of the country
has resulted in the displacement of nearly 500,000 Azerbaijani citizens, the occupation of ~20% of
Azerbaijan's land, migration of human and animal populations, and the absence of sanitary-veterinary
surveillance; further exacerbating diminished control efforts.
The gender and age distribution of reported cases indicated a higher burden of disease in males
(71.8%) compared to females in all age groups. The disproportionate infection rates in males can
possibly attributed to differences in the traditional gender roles of men and women in this region. In
Azerbaijan, men tend to take on the responsibility of the handling and slaughter of animals in addition
to increased occupational hazards such as sheep herding or working in abattoirs [11]. The reporting
among age groups showed that individuals age 20 to 29 accounted for the highest percentage of cases
(43%) across all age groups and genders, which may reflect on the types of work that are available for
individuals in this age group. These finding on the age and gender distribution of cases in Azerbaijan
were very similar to those of the neighboring Republic of Georgia [33]. The increased burden of disease
in males within the Caucuses is not seen everywhere. For example, in Kampala, Uganda case reporting
indicated women represent a higher proportion of brucellosis seropositives [34] whereas in Germany
there were equal proportions of reporting between gender groups [15]. Gender differences in case
reporting between places may be due to variation in exposures related to occupation or cultural
practices such as food preparation. Additionally, there is potential reporting bias between places due to
the insidious nature of the disease as well as the utilization and access to healthcare. In Azerbaijan
gender differences in the utilization of healthcare facilities may be a source of bias in the reporting of
cases. Clark et al. [23] showed that men have a greater tendency not to use healthcare facilities in rural
areas when compared to women. However, reporting bias related to occupational exposures are more
likely to be biased towards males potentially skewing the case reporting.
The gender and age distribution of cases suggests that food-borne illness may not be the
predominant means of transmission for the disease in Azerbaijan. While food-borne illness undoubtedly
plays a role in the high incidence of the disease, the male dominated patterns of reporting and skewed
age distribution point to high levels of occupational exposure related to transmission of the pathogen.
Yet, despite the potential occupational burden of the disease efforts also need to be focused on
reducing food-borne transmission as previous research has found that the epidemiology of human
brucellosis in some areas can potentially shift away from a disease of those who directly handle animals
to a food-borne illness [8]. Several intervention strategies to reduce the burden of brucellosis among
human populations have been suggested including: increasing local knowledge of proper food handling
techniques of dairy products such as pasteurization [35], decreasing occupational exposures, and
vaccination programs aimed at reducing the prevalence of disease in livestock [36]. However, Havaas et
al. [37] noted that changing cultural and social behaviors in areas of the Trans-Caucus region would
most likely prove to be difficult and control efforts aimed at vaccinating livestock have been shown to be
the most effective [5].
Limitations
The data presented here were taken from historical records that most likely represent an under
reporting of the disease. Analyzing cases obtained from government health care facilities may have
introduced biased since individuals in more rural areas may not readily have access to care.
Additionally, those individuals in a lower socio-economic strata (SES) may not be able to afford care,
medication, or travel to government facilities. These populations within rural areas in a low SES are also
more likely to participate in an agricultural related occupation, which may thereby have resulted in
underreporting of the burden of disease in this study. Since the onset of brucellosis is often insidious the
time of diagnosis was used as the date of infection rather than the onset of the disease. Furthermore,
cases obtained from healthcare facilities may not be representative of the general population. This may
have skewed the monthly reporting of cases since the incubation period can be greater > 1 month and
an individual may not immediately seek care [7]. However, most cases occurred during the spring and
summer months and most likely did not dramatically impact cumulative or yearly incidence risk
estimates.
There are also limitations to the sensitivity of serological tests for brucellosis and no data were
available on the percentage of human cases that were confirmed on bacterial culture, the gold standard
for Brucella species. Spatial analyses were limited to district level reporting and may not reflect localized
areas of infection inside of a given district. The age of individuals also could not be tied back to their
district of origin and so no adjustments were made. While it was hypothesized that food was not the
main source of infection in Azerbaijan there were limited epidemiological data associated with cases in
this study and the data do not provide a definitive link to occupational exposure. Furthermore, the
dummy regions created to improve the synthesis of reporting for the results do not represent or
delineate national recognizable zones.
Conclusion
Recent reporting of brucellosis in Azerbaijan indicates a slight decrease in the incidence of
disease, yet without adequate livestock surveillance and veterinary health management the brucellosis
situation in Azerbaijan may fluctuate widely. The analyses carried out in this study provide a baseline
estimation of the spatial and temporal distribution of brucellosis, thereby allowing for the evaluation of
deviations from these trends, which could potentially indicate a health emergency in the population.
Additionally, the GIS mapping and statistical methodologies may provide a first step in identifying
potential problem areas associated with high disease levels. These data can inform policy for
implementing appropriate surveillance and control measures. In Azerbaijan where a majority of cases
appear to be male dominated as a result of occupational exposures implementing targeted livestock
vaccination strategies would seem to be the most prudent choice for control. Districts identified as
having high rates of the disease could be initially targeted for directed public health interventions.
Future public health management efforts and research in this region should focus on incorporating data
on livestock in conjunction with human data to better understand the transmission dynamics of
brucellosis.
References
1.
Seleem MN, Boyle SM, Sriranganathan N: Brucellosis: a re-emerging zoonosis. Veterinary
microbiology 2010, 140:392-398 %@ 0378-1135.
2.
Benkirane A: Ovine and caprine brucellosis: World distribution and control/eradication
strategies in West Asia/North Africa region. Small Ruminant Research 2006, 62:19-25.
3.
Doganay M, Aygen B: Human brucellosis: an overview. International journal of infectious
diseases 2003, 7:173-182.
4.
Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV: The new global map of human
brucellosis. The Lancet Infectious Diseases 2006, 6:91-99 %@ 1473-3099.
5.
Roth F, Zinsstag J, Orkhon D, Chimed-Ochir G, Hutton G, Cosivi O, Carrin G, Otte J: Human health
benefits from livestock vaccination for brucellosis: case study. Bulletin of the World health
Organization 2003, 81:867-876.
6.
Fosgate GT, Carpenter TE, Chomel BB, Case JT, DeBess EE, Reilly KF: Time-space clustering of
human brucellosis, California, 1973–1992. Emerging Infectious Diseases 2002, 8:672.
7.
Young EJ: An overview of human brucellosis. Clinical Infectious Diseases 1995, 21:283-289.
8.
Chomel BB, DeBess EE, Mangiamele DM, Reilly KF, Farver TB, Sun RK, Barrett LR: Changing
trends in the epidemiology of human brucellosis in California from 1973 to 1992: a shift toward
foodborne transmission. Journal of infectious diseases 1994, 170:1216.
9.
Spink WW: WHAT IS CHRONIC BRUCELLOSIS?*. Annals of internal medicine 1951, 35:358-374.
10.
WHO: Fact sheet N173. World Health Organization, Geneva, Switzerland 1997, 7.
11.
Gurbanov S, Akhmedova S: Especially Dangerous Infections in Azerbaijan. In Emerging and
Endemic Pathogens:Advances in Surveillance, Detection and Identification. Dordecht, Netherlands:
Springer; 2010: 39-43
12.
Manaseki S: Mongolia: a health system in transition. British Medical Journal 1993, 307:1609.
13.
Carrel M, Emch M, Streatfield PK, Yunus M: Spatio-temporal clustering of cholera: the impact
of flood control in Matlab, Bangladesh, 1983-2003. Health & place 2009, 15:771-782.
14.
Clennon JA, King CH, Muchiri EM, Kariuki HC, Ouma JH, Mungai P, Kitron U: Spatial patterns of
urinary schistosomiasis infection in a highly endemic area of coastal Kenya. The American journal of
tropical medicine and hygiene 2004, 70:443.
15.
Al Dahouk S, Neubauer H, Hensel A, Schöneberg I, Nöckler K, Alpers K, Merzenich H, Stark K,
Jansen A: Changing epidemiology of human brucellosis, Germany, 1962–2005. Emerging Infectious
Diseases 2007, 13:1895.
16.
Anselin L, Syabri I, Kho Y: GeoDa: An introduction to spatial data analysis. Geographical
Analysis 2006, 38:5-22.
17.
Waller LA, Gotway CA: Applied spatial statistics for public health data. Hoboken, New Jersey:
Wiley-Interscience; 2004.
18.
Anselin L: Local indicators of spatial association—LISA. Geographical Analysis 1995, 27:93-115.
19.
Ederer F, Myers MH, Mantel N: A statistical problem in space and time: Do leukemia cases
come in clusters? Biometrics 1964, 20:626-638.
20.
Jacquez G, Greiling D, Durbeck H, Estberg L, Do E, Long A, Rommel B: ClusterSeer User Guide 2:
Software for identifying disease clusters. Ann Arbor, MI: TerraSeer Press 2002.
21.
Pappas G: REVIEW ARTICLE-Medical Progress: Brucellosis. New England Journal of Medicine
2005, 352:2325-2336.
22.
Mizanbayeva S, Smits HL, Zhalilova K, Abdoel TH, Kozakov S, Ospanov KS, Elzer PH, Douglas JT:
The evaluation of a user-friendly lateral flow assay for the serodiagnosis of human brucellosis in
Kazakhstan. Diagnostic microbiology and infectious disease 2009, 65:14-20.
23.
Clark D, Ismayilov A, Bakhishova S, Hajiyev H, Nuriyev T, Piraliyev S, Bagirov S, Aslanova A,
Qasimov M, Hepburn M: Under-utilization of health care services for infectious diseases syndromes in
rural Azerbaijan: A cross-sectional study. BMC Health Services Research 2011, 11:32.
24.
Memish Z, Mah MW, Mahmoud SA, Shaalan MA, Khan MY: Brucella bacteraemia: clinical and
laboratory observations in 160 patients. Journal of Infection 2000, 40:59-63.
25.
Cooper C: The epidemiology of human brucellosis in a well defined urban population in Saudi
Arabia. The Journal of tropical medicine and hygiene 1991, 94:416.
26.
Minas M, Minas A, Gourgulianis K, Stournara A: Epidemiological and clinical aspects of human
brucellosis in Central Greece. Jpn J Infect Dis 2007, 60:362-366.
27.
Earhart K, Vafakolov S, Yarmohamedova N, Michael A, Tjaden J, Soliman A: Risk factors for
brucellosis in Samarqand Oblast, Uzbekistan. International journal of infectious diseases 2009, 13:749753.
28.
De Massis F, Di Girolamo A, Petrini A, Pizzigallo E, Giovannini A: Correlation between animal
and human brucellosis in Italy during the period 1997–2002. Clinical microbiology and infection 2005,
11:632-636.
29.
Lerman Z, Sedik DJ: Rural transition in Azerbaijan. Plymouth, United Kingdom: Lexington Books;
2010.
30.
Alibejov N, Krische S, Wesseler J: Agro-ecological zones and their impact on farm production
and farm organization after privatization in Azerbaijan. Bornimer Agrartechnische Berichte 2001:37-42.
31.
Lerman Z: Land reform, farm structure, and agricultural performance in CIS countries. China
economic review 2009, 20:316-326.
32.
Alimardanov E, Abdullayev K, Rothenberger C, Young A: Livestock services for small-scale cattle
holders in rural Azerbaijan. Small Enterprise Development 2006, 17:62-68.
33.
Akhvlediani T, Clark D, Chubabria G, Zenaishvili O, Hepburn M: The changing pattern of human
brucellosis: clinical manifestations, epidemiology, and treatment outcomes over three decades in
Georgia. BMC Infectious Diseases 2010, 10:346.
34.
Kohei M, Eric F, Charles W, Winyi K, Mark E, Susan W: Spatial epidemiology of hospitaldiagnosed brucellosis in Kampala, Uganda. International Journal of Health Geographics 2011, 10:1-9.
35.
Al-Shamahy H, Whitty C, Wright S: Risk factors for human brucellosis in Yemen: a case control
study. Epidemiology and infection 2000, 125:309-313.
36.
Zinsstag J, Roth F, Orkhon D, Chimed-Ochir G, Nansalmaa M, Kolar J, Vounatsou P: A model of
animal–human brucellosis transmission in Mongolia. Preventive veterinary medicine 2005, 69:77-95.
37.
Havas KA, Ramishvili M, Navdarashvili A, Imnadze P, Salman M: The human–animal interface of
domestic livestock management and production and its relationship to brucellosis in the country of
Georgia 2010: A rapid assessment analysis. Preventive veterinary medicine 2012, 105:10-16.
Figure 1. Total number of newly reported cases of human brucellosis seropositives by year during the
period 1995 to 2009 shown by the black bars and incidence per 100,000 indicated by the red line.
Figure 2. Map A shows Azerbaijan grouped into dummy regions: Nakchivan (light blue), West (dark
green), Central (dark blue), and East (light green). Map B displays the total number of human brucellosis
seropositives by district during the time period 1995 to 2009 with the Central region overlain with crosshatching. The capital Baku is represented by a star in map B.
Figure 3. Spatial distribution of cumulative incidence estimates during the study period 1995 to 2009.
Cumulative estimates were calculated for three equal 5-year periods. The maps display period 1 (1995
to 1999), period 2 (2000 to 2004), and period 3 (2005 to 2009). Insets A refer to crude cumulative
incidence estimates for each time period and insets B refer to Empirical Bayes smoothed (EBS) estimates
for each time period. Cross-hatching overlain on maps depicts the Central dummy region of Azerbaijan.
During period 1 cumulative incidences ranged between 0 to 415.0 cases per 100,000 persons with the
highest rate occurring in Bilasuvar (95%CI : 368.9, 465.4), period 2 cumulative incidences that ranged
between 0 to 386.5 cases per 100,000 persons with the highest rate occurring in Gobustan (95%CI :
324.5, 456.9), and period 3 Cumulative rates during period 3 ranged between 0 to 283.4 cases per
100,000 persons with the highest incidence again occurring in Gobustan (95%CI : 232.2, 342.8).
Figure 4. Figure 4. Boxplots are displaying the crude cumulative incidence rates per district in each of the
time periods, [period 1 (1995 to 1999), period 2 (2000 to 2004), and period 3 (2005 to 2009)] paneled by
the dummy region: Central, East, Nakchivan, and West. Crude estimates were calculated for each period
using the total number of reported cases during a 5-year period as the numerator and the median year
population as the denominator.
Figure 5. Local Moran’s I clusters across Azerbaijan with red portraying High-High areas, dark blue LowLow areas, light blue Low-High areas, and pink High-Low areas. During period 1 (1995 to 1999) HighHigh clusters were identified in Gor (Goranboy), Imi (Imishli), Kha (Khanlar), Saa (Saatly), and Tar
(Tartar). During period 2 (2000 to 2004) Abs (Absheron) was identified as a High-High cluster and Khi
(Khizi) was identified during period 3 (2005 to 2009). The star represents the location of the capital
Baku and cross-hatching represents the Central region.
Figure 6. Total number of human brucellosis cases by month and season. Winter months (n= 1,000) are
shown in the graph by cross-hatched bars, Spring months (n=2,228) are displayed by the solid black bars,
Summer months (n=3,131) are represented by dotted bars, and Fall months (n=1,624) are displayed by
the solid grey bars.
Figure 7. Results from the Ederer-Myer-Mantel's (EMM) test displaying significant temporal clusters
during period 1 (1995 to 1999) shown in light grey, period 2 (2000 to 2004) shown in dark grey, and
period 3 (2005 to 2009) shown in black.
Figure 8. Age distribution of human brucellosis cases in men illustrated by the black bars and women
displayed by the grey bars.