Cytomechanical forces
advertisement
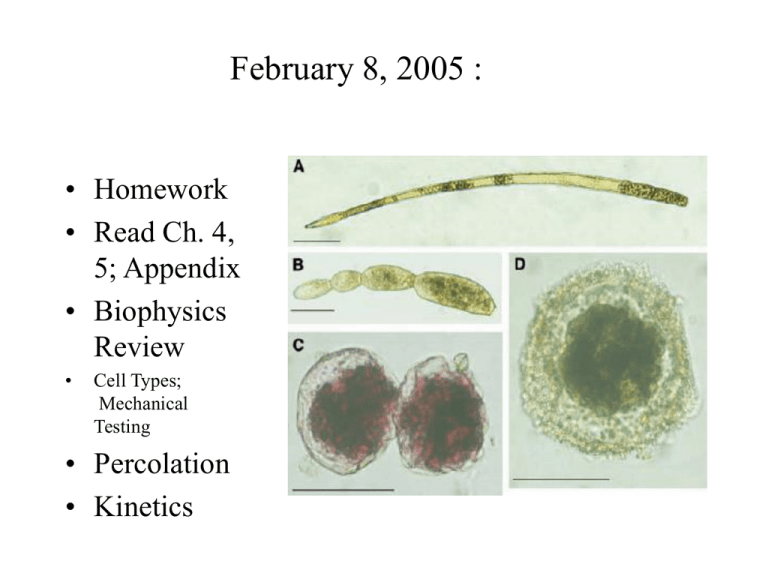
February 8, 2005 : • Homework • Read Ch. 4, 5; Appendix • Biophysics Review • Cell Types; Mechanical Testing • Percolation • Kinetics Prokaryotes • Most have elevated osmotic pressure, I.e a few tens of atmospheres. • Challenger Deep sea dropped to 10,896 m in last 8 million years (Deepest place in ocean) • Deficient in CaCO3 at that depth • Foraminifera (shelled protists) quickly evolved soft, non-calcareous shells • Likely are highly pressurized. Biomembrane as an isotropic material Bilayer compression resistance, KA = 4 g g= 0.04 J/M2 (g = surface tension) Homogeneous lipid sheet: Biomembrane A A Tm = K A = •10 atm = 106 J/m3 Stretching membrane thins it exposing hydrophobic core to Water. Rupture occurs at 210% area expansion, so say lysis tension ~ 0.016 J/M2. For a 1 mm cell : P= 64,000 J/M3 ~ 0.6 atm. at rupture. Life @ 1,200 atmoshperes • How thick does the membrane need to be? • How thick does the wall need to be? • Compressibility properties: KV d p KA = 4 / 9 KV / m KV 3m = E K A KV d p Cell Walls for strength How thick does wall need to be to withstand normal pressures inside a bacterium, I.e. 30-60 atm. ? Lets say lysis occurs when wall tension exceeds 5% of KA. We can approximate KA by KVd, and for isotropic wall material, Kv ~ E, so, assume a material E= 3 x 109 J/m3 tfailure= 0.05 KA= RP/2=0.05 E d So to not fail, d> RP/E 0.5 x10 6 106 d So for R = 0.5 mM, P= 10 atm, 3x109 5 = nM 3 Thick wall sphere Thick walled sphere • Equilibrium P • Pressure inside 2 2 ri pi ri = pi 2 = • Average stress in wall 2 h ( r r ) (ro ri ) o i (ro 2 ri 2 ) Pin = ri pi 2 in 2 Pout po ro = h(ro ri ) Pin Pout = r pi 2h r po 2h • Pressure from outside • Pressurized both sides Alternate method (not too thick wall) Rp i Rp o = 2h 2h fail = E max Laplace Law for Cylinder under pressure Homework: 1. Find wall stress for cylinder. 2. Calculate stress for a foraminefora not Assuming a thin wall. Compression of a network • • A cartesian lattice of fibers (a) subject to compressive strain (b). The boundary conditions are that the angles between the segments arising from each junction are fixed at 90 and the deformation consists in movement of the junctions toward each other along the three orthogonal axes of the lattice, with no shear deformation. The junctions, however, are allowed to rotate as compression progresses. This is equivalent to the boundary conditions in Feynman et al.36 in which fiber ends are fixed but direction changes under compression. Solution to Donnan Problem • A. [ Na ]o [ K ]o [Cl ]i [ HCO3 ]i 150 = = = = [ Na ]i [ K ]i [Cl ]o [ HCO3 ]o 144 B. Cout 58 E = log( ) = 1.03mV z Cin C. [ solutes ]i = [ solutes ]i 144 4 = 114 29 A ; A = 5mM D. 1.03mV Power from electrochemical gradients (I.e batteries) Distributed Model Driving Force determined by Nernst Electrical Model of Cell Membrane Molecular model Outside Cm gK EK Inside gNa E Na I x = g x px N x ( Ex Vm ) Ca Wave in Oocyte Mechanotransduction What opens channels? Types of mechanical analysis • • • • • Kinematics - just the connections Statics- forces without motion Dynamics- forces with motion Rigid versus deformable body FBDs FEL FBL FBR FER Loading Types • • • • • • • Tension- compression Shear Reaction Traction Friction Bending Uniaxial/bi-axial Cytomechanical forces: • • • • • • Gravitational: Muscle contraction: Contact: Buoyant: Hydraulic: (Static or dynamic) Pneumatic Cell Deformation and Stiffness • Most cells are constantly deformed in vivo by both internal and external forces. • Experimental deformations can be done by poking, squishing, osmotic swelling, electrical/magnetic fields, drugs, etc. • Cells have both area and shear stiffness, mostly due to the cytoskeleton, although lipids contribute some. Material Parameters • • • • • • • • Moduli: Young’s, area, shear, bending (flexural) Stiff versus compliant Strength versus weakness Brittle versus ductile Incompressible/Compressible Failure Ultimate tensile strength Hardness: Moh’s scale Comparative Mechanical Properties Steel Wood Bone Steel Wood Bone Cells Strain Cellular ‘pre-stress’ Cells Comparative Stiffness 10000 1200 210 21 14 1 0.007 0.01 0.0002 di am on d l st ee ne bo d wo o er ru bb su e 0.0001 tis Modulus (GPa) 100 Material Elasticity • • • • • “ut tensio sic vis” Young’s Modulus: Stress over strain Shear Modulus: Related to Poisson Comparative Strains Comparative Stiffnesses Poisson’s Effect For most engineering materials, < 0.3 Materials with = 0.5 are "Incompressible." Some materials have > 1 Cauchy Strain l lo x lo v =-(.7-1)/1 = 0.3 Y ly lyo y 1 lyo 0.7 Poisson's Ratio 0 X Incompressible Means no volume change 2 swelling y x Elastic Behaviours Unixaxial stress Pressure <1 E = / < 0 KA = P/A/A 1 2 Applying forces (testing types) Uniaxial Shear Pressure Biaxial Tension or Compression Bending Twisting Testing methods Q: What are the relative resolutions? AFM Q? Why doesn’t the AFM needle poke right through? Micropipet methods A. Whole Patched Cell Micropipette Pp Qpp Micropipette Stretch Tension Pi C Mesangial Cell i Q m (K w) C o C i =constant B. Isolated Cell Stretch Solutes Pi Solutes C i (t) Qm (Kw) Mesangial Cell C o Magnetic tweezers Wang et al, Science Pulling on CSK Shear and compression Example: Blood flow forces Optical Tweezers • • • • High resolution Refractivity of bead Trapping in the beam Limited force Optical Tweezer Swelling RBCs • • Necturus erythrocytes loaded with fluo-4 (10 µM) and exposed to UV light emitted from a mercury vapor bulb and filtered through a FITC cube (400x). (A) Cells display little fluorescence under isosmotic conditions (n=6). (B) Addition of A23187 (0.5 µM) to the extracellular medium increased fluorescence under isosmotic conditions (n=6). (C) Exposure to a hypotonic (0.5x) Ringer solution increased fluorescence compared to basal conditions (n=6). (D) A low Ca2+ hypotonic Ringer solution (5 mM EGTA) did not display the level of fluorescence normally observed following hypotonic swelling (n=6). Light et al. Stimulation Protocols Impulse Step Sinusoid Ramp Magnitude TIME Figure 4.2 Modes (top) and timing protocols (lower) of force application Bone Loading Waveforms Sickle Cell: A gel problem • Single point defect causes Hbs- a polymerizing tendency in deoxygenated state • The stiff and deformed cells damage vessels • Main approaches: – 1. Controlling kinetics of polymerization – 2. Regulating stiffness (rheology) of sickle cells. Thermal shape variations Stiff Flexible (a)-(c) Serial images of a 23 mm long relatively stiff fiber. (b) and (c) are, respectively, 21.9 and 41.4 seconds after (a). There is little visible bending (see also Figure 2(a)), consistent with a long persistence length, lp . 12.0 mm. (d)-(f) Serial images of a 20 mm long ¯flexible fiber. (e) and (f) are, respectively, 51.8 and 60.8 seconds after (d). There is marked bending and a short persistence length, lp.0.28 mm (see also Figure 2(b)). The fibers undergo diffusional motion and hence are not adhering to a glass surface, rather are free in solution, a necessary condition for using statistical mechanics to obtain persistence lengths. The width of each frame is Statistics of fluctuations in 1 dimension Statistical Mechanics p = f kT R= p R p = 2 2 p = L3 L 2 48 (u ) x 2 Dilute Concentrated Semi-concentrated Floppy Chains Isotropic Rods Nema Harmonic motion (undamped) Gel motion follows simple rules Model will predict dynamic and Static equilibrium. m x = 2 PAu(t ) k ( x x0 ) mx = k ( x ) x 2 x = 0 Natural Frequency Damped Spring Viscosity & Elasticity • A complex material can be modeled as a purely viscous material combined with a purely elastic material, thus mathematically separating the viscosity of a material from its elasticity. A purely viscous component is a Newtonian fluid- it has no memory and no elasticity; it cannot deform as a solid. Cells generally behave as solid-liquid composites. V-E tools can quantify their behaviour, since the models separate viscosity from elasticity in a kind of finite element model. Maxwell Model: Differential method T = E 1/E T = E = = E d 1 d = dt E dt 1 = ( ) E Maxwell model: Laplace Method 1 1 Z ( s) = E s 1/E V = R = C Viscosity: Pascal-sec Compliance + Slipperiness Z o s o = Mechanical Impedance. For a step input s 1 / E 1 / s t=/E Gel Model • Make a complete model and label all parameters • Describe the output, relating what happens and why. • What is the time constant? • State the assumptions and simplifications Mechanical Terms Review • • • • • Statics and dynamics Kinematics and kinetics Vector and scalars Forces, resultants Deformation Classwork • Add damping to your model of cytogel • Describe how you can model thermal fluctuations in cell diameter, and list all the elements. List assumptions. • Write the model equation for the above. • Complete a simulink model of the above, and do all labelling, including all parameter values.






![Applied Strength of Materials [Opens in New Window]](http://s3.studylib.net/store/data/009007576_1-1087675879e3bc9d4b7f82c1627d321d-300x300.png)



