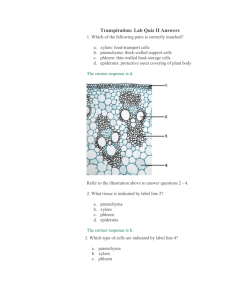
Cohesion-Tension Theory

Water In Plants
Chapter 9
Copyright © McGraw-Hill Companies Permission
Required for Reproduction or Display
Outline
•
•
•
Molecular Movement
Diffusion
Osmosis
Water Movement
Cohesion-Tension Theory
Regulation of Transpiration
Transport of Organic Solutes
Pressure-Flow Hypothesis
Mineral Requirements for Growth
Molecular Movement
•
Diffusion
Movement of molecules from a region of higher concentration to a region of lower concentration.
-
Move along a concentration gradient.
-
Move until equilibrium reached.
Diffusion
Osmosis
•
Osmosis is diffusion of water through a differentially permeable membrane from a region where the water is more concentrated to a region where it is less concentrated.
Water enters a cell by osmosis until the osmotic potential is balanced by the resistance to expansion of the cell wall.
-
Turgor Pressure
Pressure Potential
Osmosis
•
•
Water Potential of a plant is essentially its osmotic potential and pressure potential combined.
Water flows from the xylem to the leaves, evaporates within the leaf air spaces, and transpires through the stomata into the atmosphere.
In animal cells, the water potential is equal to the osmotic potential of the cytoplasm, but this is different in plant cells…
Plant cells have a cell wall, which exerts an inward pressure when the cell is turgid. This is known as the pressure potential.
The water potential of an plant cell is equal to the osmotic potential of the cytoplasm plus the cell wall pressure:
W.P.= O.P. + P.P.
Osmosis
A plant cell with water potential –50 is placed in a solution…
Water potential of cytoplasm
= -50
Osmotic potential of solution
= -20
If the solution is hypotonic, net endosmosis occurs and the cell becomes fully turgid.
Water potential of cytoplasm = -50
Osmotic potential of solution = -80
If the solution is hypertonic, net exosmosis occurs and causes plasmolysis (the cell membrane pulls away from the cell wall. The cell wall stays intact).
Water potential of cytoplasm = -50
Osmotic potential of solution = -50
If the solution is isotonic, no net osmosis occurs.
The cell is not plasmolysed, but it is not fully turgid either.
Molecular Movement
•
•
Plasmolysis
Loss of water through osmosis is accompanied by shrinkage of protoplasm away from the cell wall.
Imbibition
Colloidal material and large molecules usually develop electrical charges when they are wet, and thus attract water molecules.
Plasmolysis
Molecular Movement
•
Active Transport
Plants absorb and retain solutes against a diffusion, or electrical, gradient through the expenditure of energy.
-
Involves proton pump.
Water and Its Movement Through The Plant
•
More than 90% of the water entering a plant passes into leaf air spaces and then evaporates through the stomata into the atmosphere
( Transpiration ).
Usually less than 5% of water escapes through the cuticle.
A mature corn plant transpires about
15 liters/week
1 acre field: 1,325,000 liters/100 day growth season
Copyright © McGraw-Hill Companies Permission
Required for Reproduction or Display
Copyright © McGraw-Hill Companies Permission
Required for Reproduction or Display
Water Transport Theories
•
•
•
1682 Nehemiah Grew : Xylem Pumping:
Water also raise in dead stems.
Marcello Malphigi: Capillary action: Altough 1 mt can be reached, capillary must be open ended.
Stephen Hales: Root pressure: Only up to 30 gr/cm 2 which is not enough to carry water up to 100 mt trees.
Cohesion-Tension Theory or Transpirational Pull
Theory
Stephen Hales (1727) In his book: Vegetable
Statistics proposed the principles.
However, Hales' ideas were not understood at the time, so his findings failed to influence the debate on water transport in plants in the
19 th century.
At the begining of the 20 th century cohesion theory of water movement in plants has been ascribed to Josef
Böhm, Henry H. Dixon and John Joly and Eugen Askenasy
Cohesion - Tension Theory
•
When the negatively charged end of one water molecule comes close to the positively charged end of another water molecule, weak hydrogen bonds hold the molecules together.
Water molecules adhering to capillary walls, and each other, create a certain amount of tension.
Cohesion - Tension Theory
•
When water transpires, the cells involved develop a lower water potential than the adjacent cells.
Creates tension on water columns, drawing water from one molecule to another, throughout the entire span of xylem cells.
Theory proposes that water is pulled up xylem by the surface tension generated at the interface between the atmosphere and water inside the leaf.
A steep water potential gradient is created when the stomatal pore opens and the humid leaf interior is exposed to the dry air.
Water exits the leaf and menisci form at the airwater interface.
1.
2.
3.
4.
5.
6.
7.
Regulation of Transpiration:
Stomatal Conductance
The blue light at dawn is the signal that is recognized by a receptor on the guard cell.
The receptor signals the H + -ATPases on the guard cell’s plasma membrane to start pumping protons (H + ) out of the guard cell. This loss of positive charge creates a negative charge in the cell.
Potassium ions (K + ) enter the guard cell through channels in the membrane, moving toward its more negative interior.
As the potassium ions accumulate in the guard cell, the osmotic pressure is lowered.
A lower osmotic pressure attracts water to enter the cell.
As water enters the guard cell, its hydrostatic pressure increases.
The pressure causes the shape of the guard cells to change and a pore is formed, allowing gas exchange.
Regulation of Transpiration:
Factors
•
•
Stomata of most plants are open during the day and closed at night.
Stomata of many desert plants open only at night.
-
Conserves water, but makes carbon dioxide inaccessible during the day.
Humidity plays an inverse role in transpiration rates.
High humidity reduces transpiration, while low humidity accelerates it.
GUTTATION
•
If a cool night follows a warm, humid day, water droplets may be produced through hydathodes at the tips of veins of some plants.
Copyright © McGraw-Hill Companies Permission Required for Reproduction or Display
Measurements of water potential
Thermocouple psychrometer
The pressure chamber
A pressure chamber measures the tension
(Y p
) in xylem
Air pressure in the chamber
“squeezes” on the leaf tissues, forcing water out of leaves, into xylem, and out of the cut stem
To understand how to interpret measurements from pressure chambers you need to know:
FIRST: a. Water in the xylem is under tension . The pressure potential is negative. b. Solute potential in the xylem is usually close to zero c. So – the total water potential of the xylem is approximately equal to the pressure potential:
Y w
(xylem)
Y p
(xylem)
SECOND:
In equilibrium conditions, the total water potential of xylem
(dead cells) is equal to the water potential of living cells surrounding it
Y w is the same everywhere
If the solute potential (Y s
) of the xylem is close to zero, then the pressure chamber measures xylem water potential, which is in equilibrium with the water potential of leaf mesophyll cells:
P chamber
:
= - Y p
- Y w
- Y w
(xylem)
(xylem)
(leaf mesophyll)
Transport of Organic Solutes in Solution
•
One of most important functions of water in the plant involves the translocation of food substances in solution by the phloem.
Most of our knowledge on this subject came from studying aphids feeding on phloem.
Pressure-Flow Hypothesis
•
•
Organic solutes flow from a source where water enters by osmosis.
Organic solutes are moved along concentration gradients between sources and sinks .
High sugar concentration in phloem sap at source leads to movement of water from xylem into phloem increasing turgor pressure.
Sugars are transported from source to sink along this turgor pressure gradient in phloem.
-
Low sugar concentration in phloem sap at sink reduces turgor pressure and water moves from phloem sap to xylem.
-
Water flows in continuous loop driven by water potential gradients between xylem and phloem.
-
Sugars move in one direction by bulk flow along turgor pressure gradient in phloem.
Sugar Loading and Unloading
Sucrose is transported into phloem cells at source against a concentration gradient and requires energy.
Two membrane proteins, a proton pump and a proton – sucrose cotransporter, are involved in phloem loading.
Plant Nutrition
•
Essential plant nutrient
An element functions in the metabolism of a plant and the plant cannot complete its life cycle without the element.
Macronutrients and Micronutrients
•
•
Macronutrients are used by plants in greater amounts.
Nitrogen, potassium, calcium, phosphorus, magnesium, and sulfur.
Micronutrients are needed by the plants in very small amounts.
Mineral Requirements for Growth
•
Essential Elements
Plant Nutrition
•
Essential plant nutrients
Essential Element
Hydrogen
Oxygen
Carbon
Nitrogen
Potassium
Calcium
Magnesium
Phosphorus
Sulfur
Chlorine
Iron
Boron
Manganese
Zinc
Copper
Molybdenum
Available Form(s)
H
2
O
CO
2 and H
2
O
CO
2
NO
3
and NH
4
+
K +
Ca 2+
Mg 2+
H
2
PO
4
and HPO
4
2-
SO
4
2-
Cl -
Fe 2+ and Fe 3+
H
3
BO
3
Mn 2+
Zn 2+
Cu + and Cu 2+
MoO
4
2-
Table 1-8 Textbook
Relative
Concentration (ppm)
60,000,000
30,000,000
30,000,000
1,000,000
400,000
200,000
100,000
30,000
30,000
3,000
2,000
2,000
1,000
300
100
1
C . H O P K i N S CaFe M ighty g ood CuZn , B urley Mn ager, Mo tley Cl erk
Plant Nutrition
Nitrogen
(N)
1 - 5% N
50-500 lb/A
•
•
•
•
•
•
• adsorbed as both nitrate
(NO
(NH
3
-
4
+
) and ammonium
) component of amino acids and proteins component of nucleic acids
(DNA and RNA) component of chlorophyll many enzymes contain N continuously reused as proteins are broken down and resynthesized mobile in the plant
Plant Nutrition
Phosphorus (P)
•
0.1 -0.5% P
•
30 – 175 lb/A
(P
2
O
5
)
•
•
•
•
• adsorbed as H
2
PO
4
and
HPO
4
2important in energy storage and transfer (ADP and
ATP) component of nucleic acids
(DNA and RNA) component of phosphoproteins and phospholipids many enzymes contain P important in root growth and seed (grain) production mobile in the plant
Plant Nutrition
Potassium (K)
0.5 – 6 %
50 – 500 lb/A
•
•
•
•
•
•
• adsorbed as K + important in plant water uptake and balance through effect on osmotic potential cation balance for anion transport cofactor for many enzymes used in many process such as synthesis of proteins, ATP and in photosynthesis however not a constituent of any compounds mobile in the plant
Plant Nutrition
Calcium
(Ca)
0.2 – 1%
10 -175 lb/A
•
•
•
•
•
• adsorbed as Ca 2+ component of cell membranes and cell walls (calcium pectate) important for nutrient uptake important for cell elongation and division cation balance for anion transport immobile in the plant
Magnesium
(Mg)
0.1 – 0.4 %
10 – 175 lb/A
Plant Nutrition
•
•
•
•
• adsorbed as Mg 2+ component of chlorophyll activates many enzymes component of ribosomes thus important for protein synthesis mobile in the plant
Sulfur
(S)
0.1-0.5 %S
10 - 80 lb/A
Plant Nutrition
1.
2.
3.
4.
5.
adsorbed as SO
4
2component of amino acids
(cysteine and methionine) and thus proteins important in synthesis of vitamins, hormones, and other plant metabolites component of glycocides which give odor to onions, mustard, etc.
Immobile in the plant
Plant Nutrition
Boron
(B)
Monocots
6 - 18 ppm
Dicots
20 – 60 ppm
•
•
•
•
• adsorbed as boric acid
(H
3
BO
3
) small amounts as dissociated ionic borates involved in transport of sugars across cell membranes and in carbohydrate metabolism important in cell development and elongation important in nodulation in legumes immobile in the plant
Plant Nutrition
Iron
(Fe)
10 – 1000 ppm
•
•
•
•
•
• adsorbed as Fe 2+ and
Fe 3+ may also be adsorbed as organically complexed
Fe (chelates) involved in redox reactions in cells involved in photosynthesis involved in chlorophyll and protein synthesis important in respiratory enzymes immobile in the plant
Plant Nutrition
Manganese
(Mn)
20 – 500 ppm
•
•
•
•
• adsorbed as Mn 2+ involved in redox reactions in cells involved in photosynthesis
(formation of O
2
) can substitute for magnesium in activating many enzymes immobile in the plant
Copper
(Cu)
5 – 20 ppm
Plant Nutrition
•
•
•
•
• adsorbed as Cu 2+ involved in redox reactions in cells activates many enzymes involved in cell wall formation immobile in plants
Plant Nutrition
Zinc
(Zn)
25 – 150 ppm
•
•
•
• adsorbed as Zn 2+ involved in enzyme synthesis and activation component of auxin
(growth regulator) immobile in plants
Plant Nutrition
Molybdenum
(Mo)
< 1 ppm
•
•
•
• adsorbed as MoO
4
2component of enzymes systems important in the reduction of NO
3 and in N legumes
2 to NH fixation by
4 involved in Fe adsorption and transport immobile in the plant
Plant Nutrition
Chlorine
(Cl)
0.2 – 2 %
•
•
•
•
•
• adsorbed as Cl involved in photosynthesis plays role with K in the water balance of the plant not a constituent of any compounds may be important in disease resistance mobile in the plant
Review
•
•
•
Molecular Movement
Diffusion
Osmosis
Water Movement
Cohesion-Tension Theory
Regulation of Transpiration
Transport of Organic Solutes
Pressure-Flow Hypothesis
Mineral Requirements for Growth
Copyright © McGraw-Hill Companies Permission Required for Reproduction or Display