Biology Unit 1 Review Worksheet
advertisement

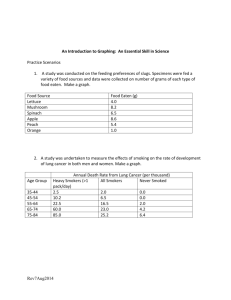
Unit 1 Review 1. In the following situation, identify the hypothesis, independent variable, dependent variable, the control and constants. After studying about recycling, members of John’s biology class investigated the effect of various recycled products on plant growth. John’s lab group compared the effect of different aged grass compost on bean plants. Because decomposition is necessary to release the nutrients, the group hypothesized that older grass compost would produce taller bean plants. Three flats of bean plants (25 plants/ flat) were grown for 5 days. The plants were fertilized as follows: (a) Flat A: 450 g of three-month-old compost, (b) Flat B: 450 g of six-month-old compost, and (c) Flat C: 0 g compost. The plants received the same amount of sunlight and water each day. At the end of the 30 days the group recorded the height of the plants (cm). Hypothesis: ____________________________________________________________________________ ______________________________________________________________________________________ IV: ___________________________________________________________________________________ DV: __________________________________________________________________________________ control: _______________________________________________________________________________ constants: _____________________________________________________________________________ 2. A team of scientists wanted to test the effects of temperature on the germination rate of pinto beans. They placed three sets of 100 pinto bean seeds in temperature controlled chambers: Chamber A was set at 15 o C, chamber B at 20oC, and chamber C at 25oC. Their results are shown in Table 1 below: Germination Rates of Pinto Beans % Germination % Germination % Germination (15o C) (20o C) (25o C) 0 0 0 0 2 2 10 10 4 10 30 50 6 20 40 80 8 20 60 90 10 35 70 90 Day A. Identify the Independent Variable: _________________________________________ B. Identify the Dependent Variable: __________________________________________ 3. Identify the following situations as one of the 8 characteristics of life. a) a cell divides b) a giraffe eats the leaves off of a tree c) when looking thru a microscope at a sample of elephant skin, you see thousands of cells d) a human being gets goose bumps and shivers when it’s cold outside to help stay at the same internal temperature e) a plant captures the sun’s rays to make glucose f) a sperm and an egg meet to create an embryo g) A rabbit’s fur turns white in the winter and brown in the summer 4. Ionic bonding occurs between a ____________________ element and a ________________________ element. Describe what happens to the valence electrons in an ionic bond. 5. Covalent bonding occurs between a ____________________ element and a ____________________ element. Describe what happens to the valence electrons in a covalent bond. 6. What makes carbon such a unique atom in molecules of living things? 7. Molecules that contain carbon are called ______________________________. 8. Draw and label water molecules to show their polarity (+) and (-) and hydrogen bonding. 9. What advantage is it that water behaves this way to nature? 10. Identify each statement below as true or false. a. _____ In a water molecule, electrons are shared equally between the hydrogen atoms and oxygen atom. b. _____ Because of its polarity, water can move from the roots of a plant up to its leaves. c. _____ Water changes temperature easily. d. _____ Unlike most substances, water expands when it freezes. 11. Water’s specific heat is _____________ (high or low?). This means it takes a ____________ time to heat water up and a ___________ time to cool water down. 12. Which food molecule (monosaccharide, polysaccharide, lipid, protein) would you eat if… a) …you needed a quick boost of energy? b) …you wanted to grow strong nails? c) …you haven’t eaten in days? d) …you wanted to grow healthy hair? e) …you had a race tomorrow afternoon? f) …you were getting ready for hibernation? g) …you wanted to get bigger muscles? 13. What is a chemical reaction? What are reactants? What are products? 14. Label parts a – e on the graph 15. What is happening at b? 16. What is the relationship between the energy of the reactants and the energy of the products? 17. Define activation energy. 18. Which letter represents the activation energy for the reaction? a. Without the enzyme?____ b. With the enzyme? ______ 19. What does letter e represent? 20. What is the role of enzymes in biological systems? 21. Describe active site and substrate. 22. The diagram below represents a human enzyme and four types of molecules present in a solution in a flask. Which molecule would most likely react with the enzyme? ____________ Explain why? 23. Match the enzymes with their substrates and functions. _____ _____ _____ _____ _____ _____ A. amylase B. protease C. lactase D. DNA polymerase E. maltase F. ATP synthase 1. 2. 3. 4. 5. 6. synthesizes DNA digests sugar in beer (maltose) digests starch (amylose) synthesizes ATP digests milk sugar (lactose) digests proteins Base your answers to the following questions on the graphs below and on your knowledge of enzymes. 24. What is the optimal pH for pepsin?__________ Is this pH acid or basic?___________________ 25. In what organ of the digestive system does pepsin work? __________________________ 26. What is the optimal pH for trypsin? __________ 27. In what organ of the digestive system does trypsin work? __________________________ Is this pH acid or basic? __________________ 28. What most likely happens to the rate of reaction of a human enzyme when the temperature is increased gradually from 10°C to 30°C? Explain your answer. 29. What most likely happens to the rate of reaction of a human enzyme when the temperature is increased gradually from 40°C to 90°C? Explain your answer. This does not include everything that will be on the test. Remember you will have to label an amino acid and a nucleotide, tell the monomers of all macromolecules and give the names of the reactions that are used to build them and tear them apart, know the properties of water and examples.