ATOMIC SPECTRA Objectives
advertisement
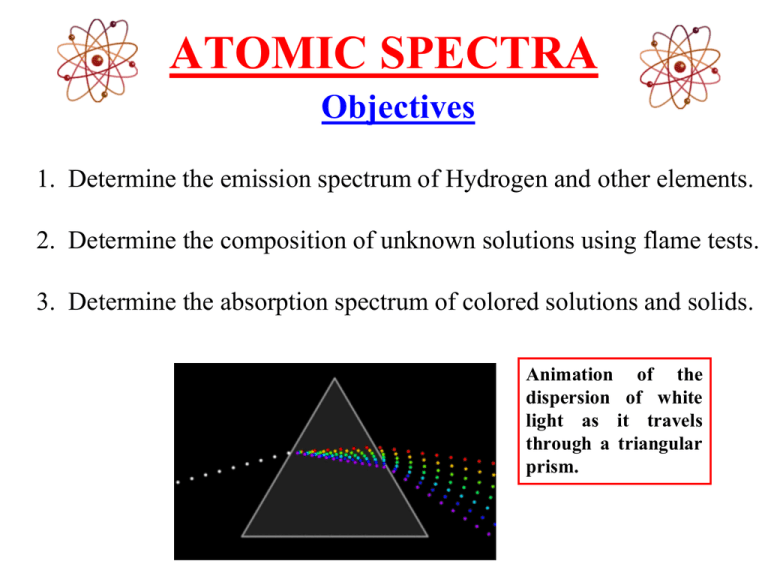
ATOMIC SPECTRA Objectives 1. Determine the emission spectrum of Hydrogen and other elements. 2. Determine the composition of unknown solutions using flame tests. 3. Determine the absorption spectrum of colored solutions and solids. Animation of the dispersion of white light as it travels through a triangular prism. A Quantitative Study of Light Sir Isaac Newton was one of the first people to study light scientifically. In 1672, Newton directed a beam of white light through a triangular bar of glass, called a “prism”. He discovered that the light coming out of the prism was separated into bands of colors. Sir Isaac Newton 1643 - 1727 The arrangement of colors produced by a prism is called a “spectrum”. Prior to this it was believed that Original Studies Of Light Used Only One Prism . When a narrow band of light from a “white” light source is sent through a prism, a continuous spectrum containing all wavelengths of visible light is formed. Newton’s Contribution to Spectroscopy Newton contributed more to spectroscopy than scientifically proving that sunlight traveling through a prism was always broken down into the components of the rainbow. In fact, his main contribution was to show that after the sunlight had been broken down into its components by one prism, if a narrow ray of the light from the first prism was passed through another prism there would be no further breakdown. Classification of Electromagnetic Radiation The color components of light are separated along the visible range of light. The visible range of light (400-700 nm) is merely a small portion of the entire electromagnetic spectrum. Advancements in the Study of Light Joseph von Fraunhofer is best known for his discovery of the dark absorption lines known as Fraunhofer lines in the Sun's spectrum, and for designing achromatic telescope objectives. At age 11, he was orphaned and forced to apprentice for no pay for a harsh glassmaker named Philipp Anton Weichelsberger. In 1801, the glass shop collapsed and Fraunhofer was buried alive. Joseph von Fraunhofer (March 6, 1787 – June 7, 1826) German Optician When Fraunhofer survived the collapse, the court-councilor von Utzschneider, gave him books on mathematics and optics. King Max Joseph also took an interest in him and gave him a present of eighteen ducats. With this money Joseph acquired his own glass grinding machine and bought his release from Development of the Spectroscope Joseph von Fraunhofer’s initial desire was to create a glass lens that did not produce an image that was fringed with a rainbow of colors. He realized the problem was that the glass lens bent some colors more than others. He began searching for a source of light of a single color. Joseph von Fraunhofer (March 6, 1787 – June 7, 1826) In 1814, he developed a spectroscope to study the spectrum of the light given off by the sun. He was amazed to discover that in the midst of the rainbow of colors was a series of black lines. These dark lines were later determined to be the result of the absorption of selected frequencies of the electromagnetic radiation Development of Diffraction Gratings Fraunhofer also completed an important theoretical work on diffraction and established the laws of diffraction. One important innovation that Fraunhofer made was to place a diffraction slit in front of the objective of a measuring telescope in order to study the solar spectrum. He later made and used diffraction gratings with up to 10,000 parallel lines per inch. By means of these gratings he was able to measure the minute wavelengths of the different colors of light. (Diffraction gratings will be discussed more later.) 1855-1860 - Gustav Kirchhoff and Robert Bunsen Gustav Robert Kirchhoff Robert Wilhelm Eberhard Bunsen (March 12, 1824 – October 17, 1887) German Physicist (March 31, 1811 – August 16,1899) German Chemist Bunsen and Kirchhoff further developed the spectroscope by incorporating the Bunsen burner as a source to heat the elements. In 1861, experiments by Kirchhoff and Bunsen demonstrated that each element, when heated to incandescence, gave off a characteristic color of light. When the light was separated into its constituent wavelengths by a prism, each element displayed a unique pattern or emission spectrum. Emission Spectra Complement Absorption Spectra The emission spectrum seemed to be the complement to the mysterious dark lines (Fraunhofer lines) in the sun's spectrum. This meant that it was now possible to identify the chemical composition of distant objects like the sun and other stars. They concluded that the Fraunhofer lines in the solar spectrum were due to the absorption of light by the atoms of various elements in the sun's atmosphere. Atomic Spectra Experiment •PART A: Hydrogen emission spectrum. •PART B: Emission spectrum of other elements. •PART C: Flame Tests (organic & inorganic). •PART D: Absorption spectrum of colored solutions and solids. PART A: Record Hydrogen line spectrum with a Scanning Spectrophotometer. The hydrogen line spectrum contains only a few discrete wavelengths. In the visible region, there are only four wavelengths. Scanning Spectrophotometer (side view) A light beam enters the spectrophotometer. The focal point of the beam is brought to the slit of the spectrophotometer. The light passing through the slit is reflected off of a collimating mirror and sent to the diffraction grating. The diffraction grating disperses the parallel beams of light into their component wavelengths. Each different wavelength comes off of the grating at a slightly different angle. So the image of the slit is spread out by color similar to a rainbow. Scanning Spectrophotometer (top view) A hydrogen light source will be viewed using a scanning spectrophotometer. The wavelengths will be calculated for the Balmer and Lyman series and then compared to those generated by the computer attached to the scanning spectrophotometer. Computer Output from a Scanning Spectrophotometer The peaks on the spectrograph correspond to the energy changes of the electrons for the Hydrogen atom. Atomic Spectra of Noble Gases Helium Neon Argon The Atomic Spectra will be determined for Hydrogen & the Noble Gases by looking at the gas discharge tubes. PART C: Flame Test (Organic Compounds) Beilstein Test If a clean copper wire is coated with a halogen-containing compound and placed in a flame, the presence of the halogen is revealed by a green to blue color. It is often possible to distinguish between chlorine, bromine and iodine based on the color of the flame. PART C: Flame tests and identification of an unknown metal. Observe and record the color of the flame for each known sample. Then determine the unknown compound based on the comparison between its flame color and those of the known samples. Flame Tests • Flame Test: A test used in the identification of certain elements. • It is based on the observation that light emitted by any element gives a unique spectrum when passed through a spectroscope. Flame spectrum for lithium. (Notice the faint bands of color in the spectra.)