ppt - Fusion Energy Research Program
advertisement

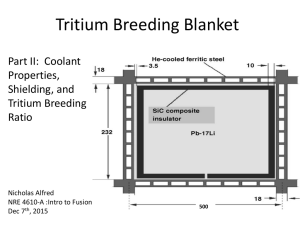
Opportunities for research on material compatibility and tritium behavior at the STAR laboratory Pattrick Calderoni Fusion Safety Program Idaho National Laboratory, USA HAPL Program Meeting GA San Diego 8-9 August 2006 Fusion Safety Program Objectives Introduce the INL Safety and Tritium Applied Research facilities and research capabilities in the areas of compatibility and tritium behavior for fusion chamber and blanket materials Summarize recent and ongoing activities at INL that are relevant to the HAPL program Present a preliminary plan to integrate HAPL chamber and blanket R&D with the current and planned STAR activities Collect directives, comments, suggestions, impressions, desires, … on technical and programmatic aspects to consolidate the preliminary plan into an R&D proposal Slide 1 Fusion Safety Program STAR Mission and Research • Provide laboratory infrastructure to study tritium science and technology issues associated with the development of safe and environmentally friendly fusion energy • Designated a National User Facility • Research thrust areas – Plasma-material interactions of PFC materials with energetic tritium and deuterium ions – Fusion safety: chemical reactivity, activation product mobilization and dust/debris characterization for PFC materials; tritium behavior in fusion systems (in-vessel source term) – Molten salts and fusion liquids for tritium breeder and coolant applications – Fission reactor tritium production permeation issues; AGR fuel tritium retention and release studies – Tritium plant and fuel cycle issues for MFE and IFE Slide 2 Fusion Safety Program STAR Floor-plan Layout Glovebox TCS Tritium SAS Chemical reactivity experiment Tritium Stack monitor D ion implantation experiment Glovebox exhaust manifold Tritium Plasma Exp Flibe-tritium experiment Flibe-corrosion experiment Flibe preparation purification & testing Flibe Salt 2Lif-BeF2 15,000 Ci tritium limit 15,000 Ci tritium limit Segregation of operations Segregation of operations/ventilation Once-through Gloveboxesroom andventilation hoods Gloveboxes and hoods Tritium cleanup system Tritium cleanup system (TCS) Once-through room Tritium storage and assayventilation system (SAS) Slide 3 Fusion Safety Program Key systems in STAR Molten Salt Tritium Behavior Experiment Tritium Plasma Experiment and Enclosure Tritium Cleanup System Molten Salt Preparation, Purification, and REDOX Experiments Tritium Storage and Assay System Steam and air chemical reactivity test apparatus Slide 4 Fusion Safety Program STAR is Ready for Operation of Tritium Experiments • Useable tritium inventory currently 1300 Ci – – – 300 Ci in equimolar H2:D2:T2 calibration standard 1000 Ci T2 available for experiments shipments from SRS limited to 1000 Ci with standard TYPE-A container • Molten Salt Tritium Permeation Experiment: – – – 100 to 300 Ci transferred as D2/T2 in vessel loaded with SAS diagnostics to include QMS, gas chromatograph, on-line ion chamber, and catalytic recovery effluent will exhaust via facility stack • TPE Tritium Experiments: – – 700 to 900 Ci transferred as T2 in vessel loaded with SAS local U-Bed capture in TPE; effluent routed to TCS for complete cleanup Slide 5 Fusion Safety Program Molten salts R&D Redox, the control of fluorine potential D. Olander, letter to the editors of J Nuc Mat (02) • Experiments at Kyoto and Tokyo Un with fast neutrons (Moriyama, Oishi 97/89, Suzuki 98/00) showed that tritium is generated in Flibe as TF without the addition of H2 • TF reacts with structural materials generating high solubility fluorides • Need to control fluorine potential to minimize corrosion • Of the three options (purge H2/HF mixtures, add metal element, add ternary salt) the use of metallic Be is best for fusion applications when considering the complexity of ternary salts chemistry and T permeation issues 0 CeF3/CeF4 NiF2 -RTlnpF2 (kJ/mole) The redox condition of molten fluoride salts is quantitatively described by the fluorine potential. The fluorine potential, however applied, controls the equilibrium concentration of structural materials dissolved in flibe. Fluoride Potential -200 H2/HF=10 FeF2 -400 H2/HF = 20/low pressure H2 -600 CrF2 Si2F6 -800 MnF2 AlF3 -1000 BeF2 -1200 LiF 450 550 650 750 Temperature (°C) Slide 6 Fusion Safety Program Molten salts R&D: redox experiments Be rod Measure HF in the gas phase as a signature of REDOX potential Inject HF • On-line detection of HF in the gas with titrator and mass spectrometer allows dynamic time dependent analysis • Controlled parameters: HF/H2 concentration, temperature, Be exposure time into the Flibe Hydrofluorination is first used to purify the salt from oxides and metal impurities: M + 2HF <--> MF2 + H2 BeO + 2HF = BeF2 + H2O When equilibrium is reached (pure salt) a metallic Be rod is inserted in the salt for a specified time Available Be reacts with HF until initial conditions are restored Slide 7 Fusion Safety Program Flibe purification facilities Hydro-fluorination approach • Control instruments & He-HF gas cabinet Bubble H2/HF/He thru melt (530ºC) Chemical Analysis of Flibe • On components • Pre and post purification • Techniques: Metals: ICP-AES, ICP-MS, acid dissolution C, N, O: LECO Pot/heater assembly Titration cell Gas manifolds HF traps O (ppm) C (ppm) N (ppm) Fe (ppm) Ni (ppm) Cr (ppm) BeF2 5700 <20 58 295 20 18 LiF 60 <20 78 100 30 4 Flibe 560 10 32 260 15 16 Processed Flibe Slide 8 Fusion Safety Program Redox experiments: analysis and complexities 1400 Simple model 1200 HF input (1000 ppm) > dissolution rate 1000 Exposure time does not influence recovery (only HF concentration) but Solubility of Be0 from integration of titrator data higher than MSRE HF concentration (ppm) Be dissolves as Be0 20 min 30 min 10 min 60 min 800 REDOX-4 REDOX-5 REDOX-6 REDOX-7 REDOX-10 600 400 200 0 Complex model 0 10 20 30 40 50 60 70 time (hr) Be dissolves as Be0 and enters the salt by galvanic mechanism as ionic Be2+ (demonstrated by recent tests with insulated Be rod) Initial ionic migration > HF input and Be deposits on Ni crucible Exposure time does not influence recovery (only HF concentration and available deposition surface for ions) Currently measuring ionic migration by electrochemical analysis to decouple processes Slide 9 Fusion Safety Program Ferritic steel corrosion tests He He+H2+HF TC He He He He+H2+HF FSCT #1 and #2 concluded Analysis of results ongoing at INL and in Japan - stay tuned for Jupiter II final reports Slide 10 Fusion Safety Program Tritium permeation experiments Experiments are designed to investigate tritium behavior in flibe / Ni systems with Redox control Previous experiments in Japan under irradiation complicated by oxide layer formation Chamber designed and constructed in Japan, tested with H2 to verify negligible convection effects Gas supply system, glove-box, instruments and control already tested with previous D2 permeation experiment 6.35 30 30 6.35 6.35 lid ss316 12.7 6.35 108 20 150 1 12.7 110 6.35 2 6.35 ss316 Ni 160 2 240 ss316 Ni 100 9.52 9.52 60.3 Ni 2 4 4 10 2 2.8 Ni 114 4 95 Slide 11 Fusion Safety Program Tritium permeation experiments • Tritium provided in pressurized vessel containing D2/T2 mixture • Glovebox setup to contain potential leaks, connected with tritium clean-up system • GC column coupled with ionization chamber has been tested with tritium • Develop DF/TF generator to compare with T2 permeation results • Builds on success of TMAP modeling with D2 permeation experiment • 1-D axial model with sink terms to simulate radial loss Vacuum pump Pressure gauge HF trap Flow meter exhaust Flow meter Gas chromatograph + ionization chamber And QMS Flow meter Cap High temperature salt Flibe Ar D2 Ni T2 Be insertion Slide 12 Fusion Safety Program Flibe and Sn-Li alloy mobilization studies for blanket safety analysis Objectives: measure the vaporization and mobilization properties of molten Sn25at%Li (in argon) and flibe (in argon, air and air+water vapor) from 600 to 1400K Approach: use a mass spectrometer equipped with a Knudsen effusion source to measure the partial pressure of condensable vapors. Partial pressures are derived from spectral line intensities after calibration with Li metal. Mobilized deposits were analyzed by ICP-AES. Slide 13 Fusion Safety Program Pb-Bi corrosion test for Fast Breeder Reactor QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. Slide 14 Fusion Safety Program Corrosion mechanism in liquid metals QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. Slide 15 Fusion Safety Program Corrosion mechanism in liquid metals QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. Slide 16 Fusion Safety Program Pb-Bi corrosion test for Fast Breeder Reactor QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. Slide 17 Fusion Safety Program Integration of HAPL chamber and blanket R&D with current and planned STAR activities A gradual integration would be beneficial to: • minimize budget for research activities and facilities • allow maximum flexibility to accommodate design changes and leverage on other R&D programs with common objectives (ITERTBM, Z-IFE, GNEP, etc) • incorporate INL Fusion Safety Program expertise in the HAPL chamber and blanket design and analysis • start a collaborative program that could lead to a full utilization of the STAR laboratory capabilities, including applied research on tritium behavior in blanket materials, tritium inventory assessment and blanket safety analysis Slide 18 Fusion Safety Program Integration of HAPL chamber and blanket R&D with current and planned STAR activities Task 1 SiC / flibe material compatibility tests and Redox control assessment and optimization Task 1 FY 1 FY2 FY3 Static compatibility test: SiC / flibe Design and construction Operation Analysis Redox and corrosion tests: SiC / flibe Design and construction Operation Analysis Flibe batch preparation Requires minimal modification of available STAR facilities for T < 700C Utilizes available state-of-the-art analytical techniques, including electrochemical measurements, and extensive expertise of scientific and technical personnel Allows comparison with static compatibility tests of Pb-17Li for TBM program ongoing at ORNL (B. Pint) Slide 19 Fusion Safety Program Integration of HAPL chamber and blanket R&D with current and planned STAR activities SiC / Pb-17Li material compatibility tests and corrosion control assessment and optimization Task 2 Task 1 FY 1 FY2 FY3 Prepare and purify Pb-17Li Design and construction Operation Analysis Corrosion control tests: SiC / Pb-17Li Design and construction Operation Analysis Requires re-assembly and modification of Pb-Bi alloy experiment Utilizes available state-of-the-art analytical techniques and expertise of scientific and technical personnel Start could wait until completion of Task 1 to leverage on other R&D and continued analysis and design of HAPL chamber and blanket system (ie, choice of coolant) Slide 20 Fusion Safety Program Integration of HAPL chamber and blanket R&D with current and planned STAR activities Task 3 Task 1 T permeation experiments in SiC / flibe systems and SiC / Pb-17Li systems FY 1 FY2 FY3 T perm test: SiC / flibe Design and construction Operation Analysis Requires modification of planned T permeation experiments in flibe / Ni systems for T < 700 C Utilizes available state-of-the-art analytical techniques and expertise of scientific and technical personnel Start would depend on Task 1 and 2 results, as well as continued HAPL blanket analysis and design (ie, coolant material choice) If comparison of material properties is needed research activities for flibe and Pb-17Li could be carried out in parallel depending on research budget and personnel availability Slide 21 Opportunities for research on material compatibility and tritium behavior at the STAR laboratory Pattrick Calderoni Fusion Safety Program Idaho National Laboratory, USA HAPL Program Meeting GA San Diego 8-9 August 2006