Speciation and reactivity of tritium in the Dart and Plym estuaries
advertisement

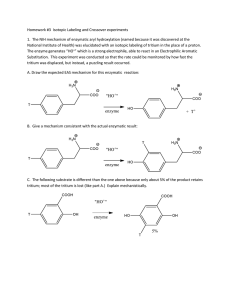
THE BEHAVIOUR OF TRITIUM IN SOUTH WEST ESTUARIES Andrew Turner, Geoffrey E. Millward & Martin Stemp School of Earth, Ocean and Environmental Sciences, University of Plymouth, Drake Circus, Plymouth PL4 8AA, UK. Tritium is a cosmogenic and anthropogenic radionuclide of hydrogen with a half-life of 12.3 years. The magnitude of liquid tritium discharges from anthropogenic sources, which exceed 1016 Bq per annum in the UK, have been based on the premise that tritium is a radioisotope of relatively low toxicity that is not concentrated in aquatic organisms or sediments, but there exists very little experimental evidence to support this assertion. To this end, we studied the speciation and sorption of tritium which was added (as tritiated water) to water samples from the Dart and Plym estuaries. The results showed that tritium rapidly equilibrates with dissolved organic ligands that are retained by a reverse-phase C18 column, and with suspended estuarine sediment particles. Significantly, a substantial fraction of sorbed tritium combined with proteinaceous material that is potentially available to sediment-feeding organisms. The extent of association of tritium with organic matter and suspended sediment far exceeds that predicted from its isotopic exchange with hydrogen, indicating that these two isotopes behave differently in aquatic environments. Tritium-hydrogen fractionation could arise because the heavy isotope preferentially enters weaker hydrogen bridges, which are characteristic of biopolymers (including natural organic substances), rather than the strong hydrogen bridges which exist between molecules of water. These characteristics have not been reported previously, but are in qualitative agreement with available measurements of tritium in estuarine and coastal waters where the principal discharge is as tritiated water. Radiological and environmental models for the transport, fate and impacts of tritium may, therefore, require revision. Corresponding author: aturner@plymouth.ac.uk