Lesson Plans Physical Science Day: Objective: D.O.L. Standard(s
advertisement

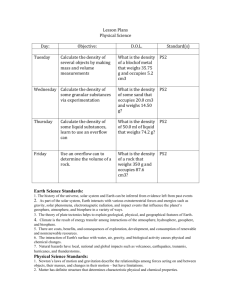
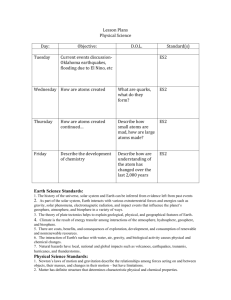
Lesson Plans Physical Science Day: Tuesday Objective: Determine the number of P,N and e- in an atom Wednesday Define isotope, Determine the number of P,N,e- in isotopes of elements D.O.L. Given any atom, and using the periodic table, determine the numbers of P,N,and e- in each atom Given masses of an atom, determine the numbers of P,N in the atom Standard(s) PS2 PS2 Thursday Define ion, Determine the number of P, e- in an atom Given the charge of PS2 an atom, determine the number of P, e- in the atom Friday Determine numbers of P,N, and e- in ions and isotopes Given the charge of PS2 an atom, or the specific isotope of an atom determine the number of P, ein the atom Earth Science Standards: 1. The history of the universe, solar system and Earth can be inferred from evidence left from past events 2. As part of the solar system, Earth interacts with various extraterrestrial forces and energies such as gravity, solar phenomena, electromagnetic radiation, and impact events that influence the planet’s geosphere, atmosphere, and biosphere in a variety of ways. 3. The theory of plate tectonics helps to explain geological, physical, and geographical features of Earth . 4. Climate is the result of energy transfer among interactions of the atmosphere, hydrosphere, geosphere, and biosphere. 5. There are costs, benefits, and consequences of exploration, development, and consumption of renewable and nonrenewable resources. 6. The interaction of Earth's surface with water, air, gravity, and biological activity causes physical and chemical changes. 7. Natural hazards have local, national and global impacts such as volcanoes, earthquakes, tsunamis, hurricanes, and thunderstorms. Physical Science Standards: 1. Newton’s laws of motion and gravitation describe the relationships among forces acting on and between objects, their masses, and changes in their motion – but have limitations. 2. Matter has definite structure that determines characteristic physical and chemical properties. 3. Matter can change form through chemical or nuclear reactions abiding by the laws of conservation of mass and energy. 4. Atoms bond in different ways to form molecules and compounds that have definite properties. 5. Energy exists in many forms such as mechanical, chemical, electrical, radiant, thermal, and nuclear, that can be quantified and experimentally determined. 6. When energy changes form, it is neither created not destroyed; however, because some is necessarily lost as heat, the amount of energy available to do work decreases. Lesson Plans Biology Day: Tuesday Objective: Review aquatic ecosystems- define estuary, intertidal zone, benthic zone, etc. D.O.L. Given a diagram label the zones of aquatic ecosystems Standard(s) LS1,LS2 Wednesday Describe how organisms are classified. Define: taxonomy, LS1,LS2 nomenclature, how a dichotomus key works Thursday Describe how a cladogram shows relationships between organisms, Describe the domains in the 5 kingdom system Explain how similar organisms are related based on amino acid similarities LS7 Friday Compare and contrast bacteria with other life forms How are viruses and bacteria different from other life forms LS7 Life Science Standards: 1. Matter tends to be cycled within an ecosystem, while energy is transformed and eventually exits an ecosystem. 2. The size and persistence of populations depend on their interactions with each other and on the abiotic factors in an ecosystem. 3. Cellular metabolic activities are carried out by biomolecules produced by organisms. 4. The energy for life primarily derives from the interrelated processes of photosynthesis and cellular respiration. Photosynthesis transforms the sun’s light energy into the chemical energy of molecular bonds. Cellular respiration allows cells to utilize chemical energy when these bonds are broken. 5. Cells use the passive and active transport of substances across membranes to maintain relatively stable intracellular environments. 6. Cells, tissues, organs, and organ systems maintain relatively stable internal environments, even in the face of changing external environments. 7. Physical and behavioral characteristics of an organism are influenced to varying degrees by heritable genes, many of which encode instructions for the production of proteins. 8. Multicellularity makes possible a division of labor at the cellular level through the expression of select genes, but not the entire genome . 9. Evolution occurs as the heritable characteristics of populations change across generations and can lead populations to become better adapted to their environment. Lesson Plans Chemistry Day: Tuesday Objective: How solubility rules are useful in determining reactions D.O.L. Determine the precipitate that forms in a double replacement reaction Standard(s) PS2 Wednesday Describe how to distinguish between silver and lead II chloride in solution Separate silver and PS2 lead II chloride compounds via experimentation Thursday Chemical reactions review, Differentiate between oxidation and reduction Given some atoms PS2 with a charge, determine which was oxidized and which was reduced Friday Balance redox reactions Given a Redox reaction, identify what is oxidized, and reduced, and balance the equation PS2 Physical Science Standards: 1. Newton’s laws of motion and gravitation describe the relationships among forces acting on and between objects, their masses, and changes in their motion – but have limitations. 2. Matter has definite structure that determines characteristic physical and chemical properties. 3. Matter can change form through chemical or nuclear reactions abiding by the laws of conservation of mass and energy. 4. Atoms bond in different ways to form molecules and compounds that have definite properties. 5. Energy exists in many forms such as mechanical, chemical, electrical, radiant, thermal, and nuclear, that can be quantified and experimentally determined. 6. When energy changes form, it is neither created not destroyed; however, because some is necessarily lost as heat, the amount of energy available to do work decreases. Lesson Plans Astronomy Day: Tuesday Objective: List and explain Newton’s Laws of motion, calculate, velocity, distance and time as appropriate Wednesday Differentiate between speed and acceleration Thursday Calculate velocity and acceleration of a toy car Friday Describe the history of manned flight, and how airplanes follow Newton’s Laws D.O.L. Standard(s) Given certain values, calculate, velocity, distance and time as appropriate PS1 Given certain values, calculate, velocity, distance, time and acceleration as appropriate Determine the acceleration, velocity of a toy car via experimentation PS1 Calculate velocity, time, distance and acceleration of airplanes PS1 PS1 Physical Science Standards: 1. Newton’s laws of motion and gravitation describe the relationships among forces acting on and between objects, their masses, and changes in their motion – but have limitations. 2. Matter has definite structure that determines characteristic physical and chemical properties. 3. Matter can change form through chemical or nuclear reactions abiding by the laws of conservation of mass and energy. 4. Atoms bond in different ways to form molecules and compounds that have definite properties. 5. Energy exists in many forms such as mechanical, chemical, electrical, radiant, thermal, and nuclear, that can be quantified and experimentally determined. 6. When energy changes form, it is neither created not destroyed; however, because some is necessarily lost as heat, the amount of energy available to do work decreases. Earth Science Standards: 1. The history of the universe, solar system and Earth can be inferred from evidence left from past events 2. As part of the solar system, Earth interacts with various extraterrestrial forces and energies such as gravity, solar phenomena, electromagnetic radiation, and impact events that influence the planet’s geosphere, atmosphere, and biosphere in a variety of ways. 3. The theory of plate tectonics helps to explain geological, physical, and geographical features of Earth . 4. Climate is the result of energy transfer among interactions of the atmosphere, hydrosphere, geosphere, and biosphere. 5. There are costs, benefits, and consequences of exploration, development, and consumption of renewable and nonrenewable resources. 6. The interaction of Earth's surface with water, air, gravity, and biological activity causes physical and chemical changes. 7. Natural hazards have local, national and global impacts such as volcanoes, earthquakes, tsunamis, hurricanes, and thunderstorms. Lesson Plans Forensic Science Day: Tuesday Objective: Identification of synthetic fibers, reaction to acetone and alcohol Wednesday Parts of a microscope, and how to use a microscope Thursday Differentiate between synthetic fibers using a microscope Friday How hair is different from artificial fibers D.O.L. Standard(s) Determine the effects of acetone, and alcohol on synthetic fibers Describe the adjustable features ( aperature, light, focusers) on a microscope, and how they can be used Demonstrate proper microscope use, make a slide using synthetic fibers Use a microscope to differentiate between different types of hair, compare and contrast hairs with other fibers Life Science Standards: 1. Matter tends to be cycled within an ecosystem, while energy is transformed and eventually exits an ecosystem. 2. The size and persistence of populations depend on their interactions with each other and on the abiotic factors in an ecosystem. 3. Cellular metabolic activities are carried out by biomolecules produced by organisms. 4. The energy for life primarily derives from the interrelated processes of photosynthesis and cellular respiration. Photosynthesis transforms the sun’s light energy into the chemical energy of molecular bonds. Cellular respiration allows cells to utilize chemical energy when these bonds are broken. 5. Cells use the passive and active transport of substances across membranes to maintain relatively stable intracellular environments. 6. Cells, tissues, organs, and organ systems maintain relatively stable internal environments, even in the face of changing external environments. 7. Physical and behavioral characteristics of an organism are influenced to varying degrees by heritable genes, many of which encode instructions for the production of proteins. 8. Multicellularity makes possible a division of labor at the cellular level through the expression of select genes, but not the entire genome . 9. Evolution occurs as the heritable characteristics of populations change across generations and can lead populations to become better adapted to their environment.