Answer Key for Atomic Timeline ( events in
advertisement
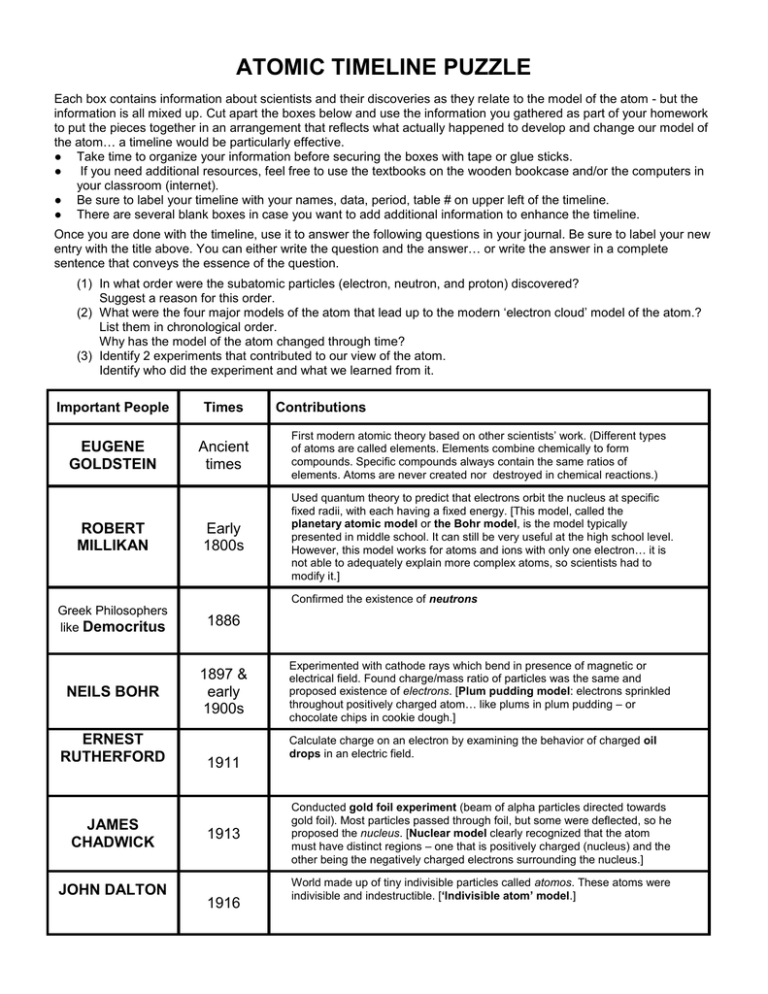
ATOMIC TIMELINE PUZZLE Each box contains information about scientists and their discoveries as they relate to the model of the atom - but the information is all mixed up. Cut apart the boxes below and use the information you gathered as part of your homework to put the pieces together in an arrangement that reflects what actually happened to develop and change our model of the atom… a timeline would be particularly effective. ● Take time to organize your information before securing the boxes with tape or glue sticks. ● If you need additional resources, feel free to use the textbooks on the wooden bookcase and/or the computers in your classroom (internet). ● Be sure to label your timeline with your names, data, period, table # on upper left of the timeline. ● There are several blank boxes in case you want to add additional information to enhance the timeline. Once you are done with the timeline, use it to answer the following questions in your journal. Be sure to label your new entry with the title above. You can either write the question and the answer… or write the answer in a complete sentence that conveys the essence of the question. (1) In what order were the subatomic particles (electron, neutron, and proton) discovered? Suggest a reason for this order. (2) What were the four major models of the atom that lead up to the modern ‘electron cloud’ model of the atom.? List them in chronological order. Why has the model of the atom changed through time? (3) Identify 2 experiments that contributed to our view of the atom. Identify who did the experiment and what we learned from it. Important People Times EUGENE GOLDSTEIN Ancient times ROBERT MILLIKAN Early 1800s Contributions First modern atomic theory based on other scientists’ work. (Different types of atoms are called elements. Elements combine chemically to form compounds. Specific compounds always contain the same ratios of elements. Atoms are never created nor destroyed in chemical reactions.) Used quantum theory to predict that electrons orbit the nucleus at specific fixed radii, with each having a fixed energy. [This model, called the planetary atomic model or the Bohr model, is the model typically presented in middle school. It can still be very useful at the high school level. However, this model works for atoms and ions with only one electron… it is not able to adequately explain more complex atoms, so scientists had to modify it.] Confirmed the existence of neutrons Greek Philosophers like Democritus 1886 NEILS BOHR 1897 & early 1900s ERNEST RUTHERFORD JAMES CHADWICK JOHN DALTON 1911 1913 1916 Experimented with cathode rays which bend in presence of magnetic or electrical field. Found charge/mass ratio of particles was the same and proposed existence of electrons. [Plum pudding model: electrons sprinkled throughout positively charged atom… like plums in plum pudding – or chocolate chips in cookie dough.] Calculate charge on an electron by examining the behavior of charged oil drops in an electric field. Conducted gold foil experiment (beam of alpha particles directed towards gold foil). Most particles passed through foil, but some were deflected, so he proposed the nucleus. [Nuclear model clearly recognized that the atom must have distinct regions – one that is positively charged (nucleus) and the other being the negatively charged electrons surrounding the nucleus.] World made up of tiny indivisible particles called atomos. These atoms were indivisible and indestructible. [‘Indivisible atom’ model.] J.J. THOMSON 1932 Found evidence of positively charged particles – protons – Looked at cathode ray tubes and noticed rays traveling in the opposite direction of the negative cathode rays. These pieces of the puzzle are from the optional names… if you use these, you will earn extra credit Proposed periodic table based on the trends of known elements. Henri Becquerel 1869 Known for discovering radioactivity while studying uranium - Isolated from the mineral pitchblende. Roentgen (RÖntgen) 1895 Suggested that an atom has a central nucleus and electrons move in orbits around the nucleus like the rings around Saturn. Hantaro Nagaoka 1896 Discovered radioactivity (the spontaneous emission of radiation) while studying mineral phosphorescence. Dmitri Mendeleev & Lothar Meyer 1896 When cathode rays strike certain materials, a new type of invisible radiation, called x-rays, is emitted. Marie & Pierre Curie 1904